Early neutropenia on day 8 treated with adjuvant Docetaxel-based chemotherapy in early breast cancer patients: Putative mechanisms within the neutrophil pool system
- PMID: 30998754
- PMCID: PMC6472781
- DOI: 10.1371/journal.pone.0215576
Early neutropenia on day 8 treated with adjuvant Docetaxel-based chemotherapy in early breast cancer patients: Putative mechanisms within the neutrophil pool system
Abstract
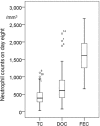
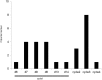
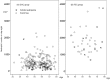
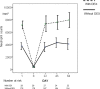
Most chemotherapy regimens cause neutropenic nadirs between days 10 and 14, and administration of granulocyte colony-stimulating factor (G-CSF) support relies on this timing. In docetaxel (DOC)-based chemotherapy, the frequency of febrile neutropenia (FN) and the G-CSF dose administered varied greatly between studies. Our study goal was to forecast the necessary dose of G-CSF by comparing day 8 neutropenia with putative changes within the neutrophil pool. We conducted a retrospective observational analysis of 242 early breast cancer patients who had received adjuvant DOC-based chemotherapy (DOC group) compared with 43 patients who had received FEC chemotherapy (FEC group). Patients who were given a standard dose and had a blood test on day 8 in the 1st cycle were eligible. In the DOC group, patients routinely received prophylactic administration of G-CSF (150 μg/body) on day 3 and received additional G-CSF based on a blood test on day 8. Results of the day 8 blood test showed that severe neutropenia (<500/mm3, average 494/mm3) was observed in 152 out of 242 (62.8%) patients in the DOC group, while in the FEC group (n = 43), neutropenia was ambiguous (average 1,741/mm3). In the FEC group, 9 out of 43 patients (20.9%) and in the DOC group, 27 out of 242 patients (11.1%) experienced FN. In the DOC group, day 8 neutropenia was predictive for FN in a logistic regression model (OR 0.79 [95% CI: 0.655-0.952], p = 0.013). Among 214 patients under 70 years old, the planned chemotherapy cycle was completed in 190 (88.8%) patients who also received the maximum dose of G-CSF (150 μg/body) four times, while 23 patients could not complete the planned chemotherapy cycle, but only five because of FN-related complications. Patients treated with DOC should be treated for primary prophylaxis with G-CSF support at an earlier time starting with a relatively small dose.
Conflict of interest statement
The author has declared that no competing interests exist.
Figures
References
-
- Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs... - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

