The possible role of cross-reactive dengue virus antibodies in Zika virus pathogenesis
- PMID: 30998804
- PMCID: PMC6472811
- DOI: 10.1371/journal.ppat.1007640
The possible role of cross-reactive dengue virus antibodies in Zika virus pathogenesis
Abstract
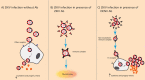
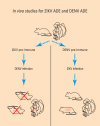
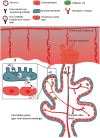
Zika virus (ZIKV) has been known for decades to circulate in Africa and Asia. However, major complications of a ZIKV infection have recently become apparent for reasons that are still not fully elucidated. One of the hypotheses for the seemingly increased pathogenicity of ZIKV is that cross-reactive dengue antibodies can enhance a ZIKV infection through the principle of antibody-dependent enhancement (ADE). Recently, ADE in ZIKV infection has been studied, but conclusive evidence for the clinical importance of this principle in a ZIKV infection is lacking. Conversely, the widespread circulation of ZIKV in dengue virus (DENV)-endemic regions raises new questions about the potential contribution of ZIKV antibodies to DENV ADE. In this review, we summarize the results of the evidence to date and elaborate on other possible detrimental effects of cross-reactive flavivirus antibodies, both for ZIKV infection and the risk of ZIKV-related congenital anomalies, DENV infection, and dengue hemorrhagic fever.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

