Quantitative atomic force microscopy provides new insight into matrix vesicle mineralization
- PMID: 30998909
- PMCID: PMC7104627
- DOI: 10.1016/j.abb.2019.04.003
Quantitative atomic force microscopy provides new insight into matrix vesicle mineralization
Abstract
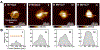
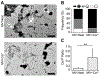
Matrix vesicles (MVs) are a class of extracellular vesicles that initiate mineralization in cartilage, bone, and other vertebrate tissues by accumulating calcium ions (Ca2+) and inorganic phosphate (Pi) within their lumen and forming a nucleation core (NC). After further sequestration of Ca2+ and Pi, the NC transforms into crystalline complexes. Direct evidence of the existence of the NC and its maturation have been provided solely by analyses of dried samples. We isolated MVs from chicken embryo cartilage and used atomic force microscopy peak force quantitative nanomechanical property mapping (AFM-PFQNM) to measure the nanomechanical and morphological properties of individual MVs under both mineralizing (+Ca2+) and non-mineralizing (-Ca2+) fluid conditions. The elastic modulus of MVs significantly increased by 4-fold after incubation in mineralization buffer. From AFM mapping data, we inferred the morphological changes of MVs as mineralization progresses: prior to mineralization, a punctate feature, the NC, is present within MVs and this feature grows and stiffens during mineralization until it occupies most of the MV lumen. Dynamic light scattering showed a significant increase in hydrodynamic diameter and no change in the zeta potential of hydrated MVs after incubation with Ca2+. This validates that crystalline complexes, which are strongly negative relative to MVs, were forming within the lumen of MVs. These data were substantiated by transmission electron microscopy energy dispersive X-ray and Fourier transform infrared spectroscopic analyses of dried MVs, which provide evidence that the complexes increased in size, crystallinity, and Ca/P ratio within MVs during the mineralization process.
Keywords: Atomic force microscopy; Elastic modulus; Matrix vesicles; Mineralization; Nucleation core.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors report no conflicts of interest.
Figures



References
-
- Bottini M, Mebarek S, Anderson KL, Strzelecka-Kiliszek A, Bozycki L, Simao AMS, Bolean M, Ciancaglini P, Pikula JB, Pikula S, Magne D, Volkmann N, Hanein D, Millan JL, Buchet R, Matrix vesicles from chondrocytes and osteoblasts: Their biogenesis, properties, functions and biomimetic models, Biochim. Biophys. Acta. Gen. Subj 1862(3) (2018) 532–546. 10.1016/j.bbagen.2017.11.005. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

