Aqueous shunts with mitomycin C versus aqueous shunts alone for glaucoma
- PMID: 30999387
- PMCID: PMC6472957
- DOI: 10.1002/14651858.CD011875.pub2
Aqueous shunts with mitomycin C versus aqueous shunts alone for glaucoma
Abstract
Background: Glaucoma affects more than 70 million people worldwide, with about 10% being bilaterally blind, making it the leading cause of irreversible blindness globally. In patients with advanced glaucoma or those who have failed medical treatment without achieving adequate intraocular pressure (IOP) control, trabeculectomy (glaucoma filtration surgery where an ostium is created into the anterior chamber from underneath a partial thickness scleral flap to allow for aqueous flow out of the anterior chamber intointo the subconjunctival space forming a filtering bleb) and aqueous shunt surgery for more complex and refractory cases remain the mainstay therapies. Proliferation of fibrous tissue around an implanted aqueous shunt may block the diffusion of aqueous humour. Mitomycin C (MMC) is one of two commonly used adjunct antifibrotic agents used during aqueous shunt surgery to prevent proliferation of fibrous tissue. However, the effectiveness and safety of the use of intraoperative MMC during aqueous shunt surgery has not been established.
Objectives: To evaluate the effectiveness and safety of MMC versus no MMC used during aqueous shunt surgery for reducing IOP in primary and secondary glaucoma.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register) (2018, Issue 2); Ovid MEDLINE; Embase.com; PubMed; Latin American and Caribbean Health Sciences Literature Database (LILACS); ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP). We did not use any date or language restrictions in the electronic search for trials. We last searched the electronic databases on 13 February 2018.
Selection criteria: We included randomized controlled trials (RCTs) in which one group of participants received MMC during aqueous shunt surgery and another group did not. We did not exclude studies based on outcomes.
Data collection and analysis: Two review authors independently reviewed titles and abstracts from the literature searches. We obtained full-text reports of potentially relevant studies and assessed them for inclusion. Two review authors independently extracted data related to study characteristics, risk of bias, and outcomes. We used standard methodological procedures expected by Cochrane.
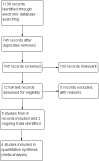
Main results: We included five RCTs, with a total of 333 eyes with glaucoma randomized, and identified two ongoing trials. All included trials examined the effect of MMC versus no MMC when used during aqueous shunt surgery for glaucoma. The trials included participants with different types of uncontrolled glaucoma. One study was conducted in China, one in Saudi Arabia, two in the USA, and one study was a multicenter study conducted in Brazil, Canada, Scotland, and USA. We assessed all trials as having overall unclear risk of bias due to incomplete reporting of study methods and outcomes; two of the five trials were reported only as conference abstracts.None of the included trials reported mean decrease from baseline in IOP; however, all five trials reported mean IOP at 12 months post-surgery. At 12 months, the effect of MMC on mean IOP compared with no MMC was unclear based on a meta-analysis of trials (mean difference -0.12 mmHg, 95% CI -2.16 to 2.41; low-certainty evidence). Two trial did not report sufficient information to include in meta-analysis, but reported that mean IOP was lower in the MMC group compared with the no MMC group at 12 months.None of the included trials reported mean change from baseline in visual acuity; however, one trial reported lower mean LogMAR values (better vision) in the MMC group than in the no MMC group at 12 months post-surgery. None of the included studies reported the proportion of participants with stable best-corrected visual acuity. Three trials reported that loss of vision was not significantly different between groups (no data available for meta-analysis).None of the included studies reported the proportion of participants with a postoperative hypertensive phase, which is defined as IOP > 21 mmHg within 3 months after surgery. Two trials reported adverse events (choroidal effusion, corneal edema, flat anterior chamber, and retinal detachment); however, due to small numbers of events and sample sizes, no clear difference between MMC and placebo groups was observed.
Authors' conclusions: We found insufficient evidence in this review to suggest MMC provides any postoperative benefit for glaucoma patients who undergo aqueous shunt surgery. Data across all five included trials were sparse and the reporting of study methods required to assess bias was inadequate. Future RCTs of this intervention should report methods in sufficient detail to permit assessment of potential bias and estimate target sample sizes based on clinically meaningful effect sizes.
Conflict of interest statement
VHXF: None known. HMH: None known. DSW: None known. SAP: None known.
Figures
Update of
- doi: 10.1002/14651858.CD011875
References
References to studies included in this review
Cantor 1998 {published data only}
-
- Cantor L, Burgoyne J, Sanders S, Bhavnani V, Hoop J, Brizendine E. The effect of mitomycin C on Molteno implant surgery: a 1‐year randomized, masked, prospective study. Journal of Glaucoma 1998;7(4):240‐6. - PubMed
-
- Cantor L, Sanders S, Bhavanani V, Hoop J, Dobler A, Harris A, et al. The effect of mitomycin‐C on Molteno implant surgery. Investigative Ophthalmology and Visual Science 1996;37:ARVO Abstract page 1162.
Costa 2004 {published data only}
-
- Costa VP, Azuara‐Blanco A, Netland PA, Lesk MR, Arcieri ES. Efficacy and safety of adjunctive mitomycin C during Ahmed Glaucoma Valve implantation: a prospective randomized clinical trial. Opthalmology 2004;111(6):1071‐6. - PubMed
-
- Costa VP, Azuara‐Blanco A, Netland PA, Lesk MR, Arcieri ES. Efficacy and safety of adjunctive mitomycin C in Ahmed Valve implantation. American Academy of Ophthalmology; 2002 Oct. 20‐23; Orlando (FL). 2002:Abstract 257. - PubMed
Duan 2003 {published data only}
-
- Duan X, Jiang Y, Qing G. Long‐term follow‐up study on Hunan aqueous drainage implantation combined with mitomycin C for refractory glaucoma. Eye Science 2003;19(2):81‐5. - PubMed
Kalenak 1996 {published data only}
-
- Kalenak J, Ripkin D, Medendorp S. A randomized controlled trial of the Molteno implant with and without mitomycin‐C. Investigative Ophthalmology and Visual Science 1996;37:ARVO Abstract page 1163.
-
- Kalenak JW, Ripkin DJ, Medendorp SV. Randomized controlled trial of the Molteno implant with and without mitomycin. American Academy of Ophthalmology; 1995 Oct 29 ‐ Nov 2; Atlanta (GA). 1995:Abstract page 136.
Sayyad 1995 {published data only}
-
- Sayyad F, Helal MM, Elsherif Z, Maghraby A. Single plate Molteno implant combined with mitomycin C (MMC) trabeculectomy in difficult glaucomas. American Academy of Ophthalmology; 1995 Oct 29 ‐ Nov 2; Atlanta (GA). 1995:Abstract page 160.
References to studies excluded from this review
Chua 2002 {published data only}
-
- Chua JK, Poon AS, Tham CC, Lai JS, Lam DS. Efficacy and safety of adjunctive mitomycin C in Ahmed Glaucoma Valve implant surgery. American Academy of Ophthalmology; 2002 Oct. 20‐23; Orlando (FL). 2002:Abstract page 257.
Cillino 2008 {published data only}
-
- Cillino S, Zeppa L, Pace F, Casuccio A, Morreale D, Bocchetta F, et al. E‐PTFE (Gore‐Tex) implant with or without low‐dosage mitomycin‐C as an adjuvant in penetrating glaucoma surgery: 2 year randomized clinical trial. Acta Opthalmologica 2008;86(3):314‐21. - PubMed
Kurnaz 2005 {published data only}
-
- Kurnaz E, Kubaloglu A, Yilmaz Y, Koytak A, Ozerturk Y. The effect of adjunctive Mitomycin C in Ahmed glaucoma valve implantation. European Journal of Opthalmology 2005;15(1):27‐31. - PubMed
Mahdy 2011 {published data only}
-
- Mahdy RA. Adjunctive use of bevacizumab versus mitomycin C with Ahmed valve implantation in treatment of pediatric glaucoma. Journal of Glaucoma 2011;20(7):458‐63. - PubMed
Yazdani 2015 {published data only}
-
- Yazdani S, Mahboobipour H, Pakravan M, Doozandeh A, Ghahari E. Adjunctive mitomycin C or amniotic membrane transplantation for Ahmed Glaucoma Valve implantation: a randomized clinical trial. Journal of Glaucoma 2016;25(5):415‐21. - PubMed
References to ongoing studies
IRCT2015101024459N1 {published data only}
-
- IRCT2015101024459N1. Evaluation the efficacy of Ahmed Glaucoma Valve implantation with and with out mitomycin C (MMC) during surgery in patients with glaucoma. en.irct.ir/trial/20639 (first received 14 January 2016).
NCT02989207 {published data only}
-
- NCT02989207. Surgical approaches in treating uncontrolled glaucoma in black African and African‐Caribbeans. clinicaltrials.gov/ct2/show/NCT02989207 (first received 12 December 2016).
Additional references
ANSI Z80.27 2001
-
- American National Standard for Ophthalmics. ANSI Z80.27 Ophthalmics ‐ aqueous shunts for glaucoma application. American Optometric Association 2001 (R2011).
Azuara‐Blanco 1997
-
- Azuara‐Blanco A, Moster MR, Wilson RP, Schmidt CM. Simultaneous use of mitomycin‐C with Baerveldt implantation. Ophthalmic Surgery and Lasers 1997;28(12):992‐7. - PubMed
Glanville 2006
GRADEpro 2015 [Computer program]
-
- GRADE Working Group, McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 7 July 2018. Hamilton (ON): GRADE Working Group, McMaster University (developed by Evidence Prime), 2015.
Green 2014
Higgins 2017
-
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Kass 2002
-
- Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open‐angle glaucoma. Archives of Ophthalmology 2002;120(6):701‐13. - PubMed
Khaw 1992
-
- Khaw P, Sherwood MB, MacKay SA, Rossi MJ, Schulz G. Five‐minute treatments with fluorouracil, floxuridine, and mitomycin have long‐term effects on human Tenon's capsule fibroblasts. Archives of Ophthalmology 1992;110(8):1150‐4. - PubMed
Kook 2000
-
- Kook MS, Yoon J, Kim J, Lee MS. Clinical results of Ahmed glaucoma valve implantation in refractory glaucoma with adjunctive mitomycin C. Ophthalmic Surgery and Lasers 2000;31(2):100‐6. - PubMed
Lee 1997
-
- Lee D, Shin DH, Birt CM, Kim C, Kupin TH, Olivier MM, et al. The effect of adjunctive mitomycin C in Molteno implant surgery. Ophthalmology 1997;104(12):2126–35. - PubMed
Meitz 1995
-
- Mietz H, Krieglstein GK. Short‐term clinical results and complications of trabeculectomies performed with mitomycin C using different concentrations. International Ophthalmology 1995;19(1):51‐6. - PubMed
Minckler 1987
Minckler 1997
-
- Minckler DS. Glaucoma drainage devices, horsehair to silicone. In: Buskirk EM, Shields MB editor(s). 100 Years of Progress in Glaucoma. Philadelphia: Lippincott‐Rave, 1997:287–92.
Minckler 2008
-
- Minckler DA, Francis BA, Hodapp EA, Jampel HD, Lin SC, Samples JR, et al: American Academy of Ophthalmology Ophthalmic Technology Assessment Committee Glaucoma Panel. Aqueous shunts in glaucoma OTA. Ophthalmology 2008;115:1089‐98. - PubMed
Perkins 1995
-
- Perkins TW, Cardakli UF, Eisele JR, Kaufman PL, Heatley GA. Adjunctive mitomycin C in Molteno implant surgery. Ophthalmology 1995;102(1):91‐7. - PubMed
Perkins 1998
-
- Perkins TW, Gangnon R, Ladd W, Kaufman PL, Libby CM. Molteno implant with mitomycin C: intermediate‐term results. Journal of Glaucoma 1998;7(2):86‐92. - PubMed
Prata 1995
-
- Prata JA Jr, Mérmoud A, LaBree L, Minckler DS. In vitro and in vivo flow characteristics of glaucoma drainage implants. Ophthalmology 1995;102(6):894‐904. - PubMed
Quigley 2006
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robin 1997
-
- Robin AL, Ramakrishnan R, Krishnadas R, Smith SD, Katz JD, Selvaraj S, et al. A long‐term dose‐response study of mitomycin in glaucoma filtration surgery. Archives of Ophthalmology 1997;115(8):969‐74. - PubMed
Rosenberg 1996
-
- Rosenberg LF, Krupin T. Implants in glaucoma surgery. In: Ritch R, Shields MB, Krupin T editor(s). The Glaucomas. St. Louis: Mosby, 1996:1783‐807.
Schocket 1986
Singh 1988
-
- Singh G, Wilson MR, Foster CS. Mitomycin eye drops as treatment for pterygium. Ophthalmology 1988;95(6):813‐21. - PubMed
Trible 1997
-
- Trible JR, Brown DB. Occlusive ligature and standardized fenestration of a Baerveldt tube with and without antimetabolites for early postoperative intraocular pressure control. Ophthalmology 1998;105(12):2243‐50. - PubMed
Tseng 2017
Wang 2013
Weinreb 2004
-
- Weinreb RN, Khaw PT. Primary open‐angle glaucoma. Lancet 2004;363(9422):1711‐20. - PubMed
Wilcox 1994
-
- Wilcox MJ, Minckler DS, Odgen TE. Pathophysiology of artificial aqueous drainage in primate eyes with Molteno implants. Journal of Glaucoma 1994;3(2):140‐51. - PubMed
Wilkins 2010
Yoon 2004
-
- Yoon PS, Singh K. Update on antifibrotic use in glaucoma surgery, including use in trabeculectomy and glaucoma drainage implants and combined cataract and glaucoma surgery. Current Opinion in Ophthalmology 2004;15(2):141‐6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical