A Review on Surface-Enhanced Raman Scattering
- PMID: 30999661
- PMCID: PMC6627380
- DOI: 10.3390/bios9020057
A Review on Surface-Enhanced Raman Scattering
Abstract
Surface-enhanced Raman scattering (SERS) has become a powerful tool in chemical, material and life sciences, owing to its intrinsic features (i.e., fingerprint recognition capabilities and high sensitivity) and to the technological advancements that have lowered the cost of the instruments and improved their sensitivity and user-friendliness. We provide an overview of the most significant aspects of SERS. First, the phenomena at the basis of the SERS amplification are described. Then, the measurement of the enhancement and the key factors that determine it (the materials, the hot spots, and the analyte-surface distance) are discussed. A section is dedicated to the analysis of the relevant factors for the choice of the excitation wavelength in a SERS experiment. Several types of substrates and fabrication methods are illustrated, along with some examples of the coupling of SERS with separation and capturing techniques. Finally, a representative selection of applications in the biomedical field, with direct and indirect protocols, is provided. We intentionally avoided using a highly technical language and, whenever possible, intuitive explanations of the involved phenomena are provided, in order to make this review suitable to scientists with different degrees of specialization in this field.
Keywords: Raman; SERS; biomedical applications; chemical enhancement; electromagnetic enhancement; enhancement factor; excitation wavelength; substrates; surface enhanced; underpotential deposition.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

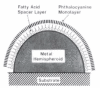
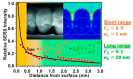
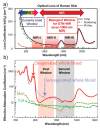

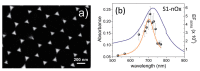






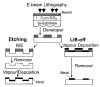
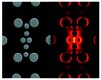



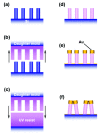

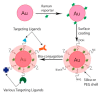








References
-
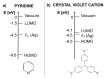
- Fleischmann M., Hendra P.J., McQuillan A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974;26:163–166. doi: 10.1016/0009-2614(74)85388-1. - DOI
-
- Jeanmaire D.L., Van Duyne R.P. Surface Raman spectroelectrochemistry. J. Electroanal. Chem. Interfacial Electrochem. 1977;84:1–20. doi: 10.1016/S0022-0728(77)80224-6. - DOI
-
- Albrecht M.G., Creighton J.A. Anomalously intense Raman spectra of pyridine at a silver electrode. J. Am. Chem. Soc. 1977;99:5215–5217. doi: 10.1021/ja00457a071. - DOI
-
- Moskovits M. Surface roughness and the enhanced intensity of Raman scattering by molecules adsorbed on metals. J. Chem. Phys. 1978;69:4159–4161. doi: 10.1063/1.437095. - DOI
-
- Moskovits M. Surface-enhanced spectroscopy. Rev. Mod. Phys. 1985;57:783. doi: 10.1103/RevModPhys.57.783. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

