Ai-lncRNA EGOT enhancing autophagy sensitizes paclitaxel cytotoxicity via upregulation of ITPR1 expression by RNA-RNA and RNA-protein interactions in human cancer
- PMID: 30999914
- PMCID: PMC6471868
- DOI: 10.1186/s12943-019-1017-z
Ai-lncRNA EGOT enhancing autophagy sensitizes paclitaxel cytotoxicity via upregulation of ITPR1 expression by RNA-RNA and RNA-protein interactions in human cancer
Abstract
Background: The biology function of antisense intronic long noncoding RNA (Ai-lncRNA) is still unknown. Meanwhile, cancer patients with paclitaxel resistance have limited therapeutic options in the clinic. However, the potential involvement of Ai-lncRNA in paclitaxel sensitivity remains unclear in human cancer.
Methods: Whole transcriptome sequencing of 33 breast specimens was performed to identify Ai-lncRNA EGOT. Next, the role of EGOT in regulation of paclitaxel sensitivity was investigated. Moreover, the mechanism of EGOT enhancing autophagy sensitizes paclitaxel cytotoxicity via upregulation of ITPR1 expression by RNA-RNA and RNA-protein interactions was investigated in detail. Furthermore, upstream transcriptional regulation of EGOT expression was also investigated by co-immunoprecipitation and chromatin immunoprecipitation. Finally, clinical breast specimens in our cohort, TCGA and ICGC were applied to validate the role of EGOT in enhancing of paclitaxel sensitivity.
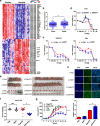
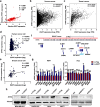
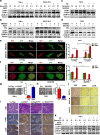
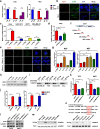
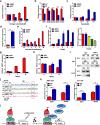
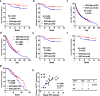
Results: EGOT enhances autophagosome accumulation via the up-regulation of ITPR1 expression, thereby sensitizing cells to paclitaxel toxicity. Mechanistically, on one hand, EGOT upregulates ITPR1 levels via formation of a pre-ITPR1/EGOT dsRNA that induces pre-ITPR1 accumulation to increase ITPR1 protein expression in cis. On the other hand, EGOT recruits hnRNPH1 to enhance the alternative splicing of pre-ITPR1 in trans via two binding motifs in EGOT segment 2 (324-645 nucleotides) in exon 1. Moreover, EGOT is transcriptionally regulated by stress conditions. Finally, EGOT expression enhances paclitaxel sensitivity via assessment of cancer specimens.
Conclusions: These findings broaden comprehensive understanding of the biology function of Ai-lncRNAs. Proper regulation of EGOT may be a novel synergistic strategy for enhancing paclitaxel sensitivity in cancer therapy.
Keywords: Ai-lncRNA; Autophagy; Cancer; EGOT; ITPR1; Paclitaxel.
Conflict of interest statement
Ethics approval and consent to participate
All patients consented to an institutional review board-approved protocol that allows comprehensive analysis of tumor samples (Ethics committee of Harbin Medical University). This study conforms to the Declaration of Helsinki.
Consent for publication
Written informed consent for publication was obtained from the patients. All authors have agreed to publish this manuscript.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Yu Y, Gaillard S, Phillip JM, Huang TC, Pinto SM, Tessarollo NG, Zhang Z, Pandey A, Wirtz D, Ayhan A, et al. Inhibition of spleen tyrosine kinase potentiates paclitaxel-induced cytotoxicity in ovarian Cancer cells by stabilizing microtubules. Cancer Cell. 2015;28:82–96. doi: 10.1016/j.ccell.2015.05.009. - DOI - PMC - PubMed
-
- Jeon YJ, Khelifa S, Ratnikov B, Scott DA, Feng Y, Parisi F, Ruller C, Lau E, Kim H, Brill LM, et al. Regulation of glutamine carrier proteins by RNF5 determines breast cancer response to ER stress-inducing chemotherapies. Cancer Cell. 2015;27:354–369. doi: 10.1016/j.ccell.2015.02.006. - DOI - PMC - PubMed
-
- Domenech E, Maestre C, Esteban-Martinez L, Partida D, Pascual R, Fernandez-Miranda G, Seco E, Campos-Olivas R, Perez M, Megias D, et al. AMPK and PFKFB3 mediate glycolysis and survival in response to mitophagy during mitotic arrest. Nat Cell Biol. 2015;17:1304–1316. doi: 10.1038/ncb3231. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

