Efficacy and safety of namilumab, a human monoclonal antibody against granulocyte-macrophage colony-stimulating factor (GM-CSF) ligand in patients with rheumatoid arthritis (RA) with either an inadequate response to background methotrexate therapy or an inadequate response or intolerance to an anti-TNF (tumour necrosis factor) biologic therapy: a randomized, controlled trial
- PMID: 30999929
- PMCID: PMC6471864
- DOI: 10.1186/s13075-019-1879-x
Efficacy and safety of namilumab, a human monoclonal antibody against granulocyte-macrophage colony-stimulating factor (GM-CSF) ligand in patients with rheumatoid arthritis (RA) with either an inadequate response to background methotrexate therapy or an inadequate response or intolerance to an anti-TNF (tumour necrosis factor) biologic therapy: a randomized, controlled trial
Abstract
Background: Namilumab (AMG203), an immunoglobulin G1 monoclonal antibody that binds with high affinity to granulocyte-macrophage colony-stimulating factor (GM-CSF), was evaluated in a phase II randomized, double-blind, placebo-controlled study to investigate the efficacy and safety in patients with rheumatoid arthritis (RA) with an inadequate response to methotrexate (MTX-IR) or anti-tumour necrosis factor therapy (TNF-IR).
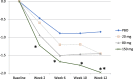
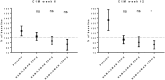
Methods: Subcutaneous namilumab (20, 80, or 150 mg) or placebo was administered at baseline and weeks 2, 6, and 10 in patients on stable background methotrexate therapy who were with MTX-IR or TNF-IR. Primary endpoint was mean change from baseline in the 28-joint Disease Activity Score, C-reactive protein version (DAS28-CRP) at week 12 comparing each of the three doses of namilumab to placebo. Safety and tolerability were assessed by adverse events (AEs) and pulmonary parameters. Results were analysed using the per-protocol population.
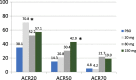
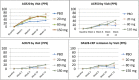
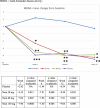
Results: One hundred eight patients from Europe and Japan (48.4 ± 12.02 years old; 77.8% female; mean DAS28-CRP 5.60-5.79; rheumatoid factor/anti-citrullinated protein antibodies + 75%) were randomized to placebo or namilumab 20, 80, or 150 mg (n = 27, 28, 25, and 28, respectively). Ninety-two were MTX-IR; 16 were TNF-IR. At week 12, a statistically significant difference in DAS28-CRP (p = 0.005) was seen for namilumab 150 mg versus placebo and separation was seen as early as week 2 for namilumab 150 mg (p < 0.05), with higher ACR50 and response rates versus placebo at week 12. A dose-response effect was observed across the DAS28-CRP endpoint with separation versus placebo evident from week 2. The most common treatment-emergent AEs were nasopharyngitis (18.5%, 17.9%, 4.0%, 14.3%), dyspnoea (0.0%, 3.6%, 8.0%, 10.7%), bronchitis (7.4%, 3.6%, 4.0%, 3.6%), and headache (3.7%, 3.6%, 12.0%, 0.0%) for placebo and 20, 80, or 150 mg of namilumab, respectively. No serious infections were observed. One serious AE (myocardial infarction) was observed with 150 mg of namilumab. There was no apparent dose relationship for AEs. A biomarker-based disease activity score showed a dose-dependent decrease at week 12.
Conclusions: This phase II study demonstrates the benefit of inhibiting macrophage activity targeting the GM-CSF for RA. The study met its primary endpoint with a clear dose-response effect. An acceptable tolerability profile was demonstrated over the 12-week study.
Trial registration: ClinicalTrials.gov, NEXUS; NCT02379091 , submitted November 28, 2014.
Keywords: GM-CSF; Namilumab; Rheumatoid arthritis.
Conflict of interest statement
Ethics approval and consent to participate
Institutional review boards or ethics committees at the participating investigational centres approved the study, which was conducted according to the principles set out in the Declaration of Helsinki, International Conference on Harmonisation Guidelines for Good Clinical Practice, and additional local regulations.
Consent for publication
Not applicable.
Competing interests
Dr. Wagner was an employee of Takeda Pharmaceuticals International GmbH. Both Bernard Souberbielle and Didier Saurigny were employees of Takeda at the time of the study. Barbara Hunt is an employee of Takeda International, Deerfield, IL, USA. The other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Similar articles
-
Phase 1b randomized, double-blind study of namilumab, an anti-granulocyte macrophage colony-stimulating factor monoclonal antibody, in mild-to-moderate rheumatoid arthritis.Arthritis Res Ther. 2017 Mar 9;19(1):53. doi: 10.1186/s13075-017-1267-3. Arthritis Res Ther. 2017. PMID: 28274253 Free PMC article. Clinical Trial.
-
MOR103, a human monoclonal antibody to granulocyte-macrophage colony-stimulating factor, in the treatment of patients with moderate rheumatoid arthritis: results of a phase Ib/IIa randomised, double-blind, placebo-controlled, dose-escalation trial.Ann Rheum Dis. 2015 Jun;74(6):1058-64. doi: 10.1136/annrheumdis-2013-204816. Epub 2014 Feb 17. Ann Rheum Dis. 2015. PMID: 24534756 Free PMC article. Clinical Trial.
-
Efficacy, pharmacokinetic, and safety assessment of adalimumab, a fully human anti-tumor necrosis factor-alpha monoclonal antibody, in adults with rheumatoid arthritis receiving concomitant methotrexate: a pilot study.Clin Ther. 2003 Jun;25(6):1700-21. doi: 10.1016/s0149-2918(03)80164-9. Clin Ther. 2003. PMID: 12860493 Clinical Trial.
-
Indirect comparisons of the efficacy of biological antirheumatic agents in rheumatoid arthritis in patients with an inadequate response to conventional disease-modifying antirheumatic drugs or to an anti-tumour necrosis factor agent: a meta-analysis.Ann Rheum Dis. 2011 Feb;70(2):266-71. doi: 10.1136/ard.2010.132134. Epub 2010 Nov 19. Ann Rheum Dis. 2011. PMID: 21097801 Review.
-
A single tumour necrosis factor haplotype influences the response to adalimumab in rheumatoid arthritis.Ann Rheum Dis. 2008 Apr;67(4):478-84. doi: 10.1136/ard.2007.074104. Epub 2007 Aug 2. Ann Rheum Dis. 2008. PMID: 17673491 Free PMC article. Review.
Cited by
-
Molecular and Cellular Heterogeneity in Rheumatoid Arthritis: Mechanisms and Clinical Implications.Front Immunol. 2021 Nov 25;12:790122. doi: 10.3389/fimmu.2021.790122. eCollection 2021. Front Immunol. 2021. PMID: 34899757 Free PMC article. Review.
-
CATALYST trial protocol: a multicentre, open-label, phase II, multiarm trial for an early and accelerated evaluation of the potential treatments for COVID-19 in hospitalised adults.BMJ Open. 2021 Nov 11;11(11):e050202. doi: 10.1136/bmjopen-2021-050202. BMJ Open. 2021. PMID: 34764169 Free PMC article.
-
Monocytes and Macrophages in Spondyloarthritis: Functional Roles and Effects of Current Therapies.Cells. 2022 Feb 2;11(3):515. doi: 10.3390/cells11030515. Cells. 2022. PMID: 35159323 Free PMC article. Review.
-
Emerging Therapeutic Options for Refractory Pulmonary Sarcoidosis: The Evidence and Proposed Mechanisms of Action.J Clin Med. 2023 Dec 19;13(1):15. doi: 10.3390/jcm13010015. J Clin Med. 2023. PMID: 38202021 Free PMC article. Review.
-
Updates on ankylosing spondylitis: pathogenesis and therapeutic agents.J Rheum Dis. 2023 Oct 1;30(4):220-233. doi: 10.4078/jrd.2023.0041. Epub 2023 Sep 6. J Rheum Dis. 2023. PMID: 37736590 Free PMC article. Review.
References
-
- Hetland ML, Christensen IJ, Tarp U, Dreyer L, Hansen A, Hansen IT, et al. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum. 2010;62:22–32. doi: 10.1002/art.27227. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

