Resolvin D1 prevents epithelial-mesenchymal transition and reduces the stemness features of hepatocellular carcinoma by inhibiting paracrine of cancer-associated fibroblast-derived COMP
- PMID: 30999932
- PMCID: PMC6472102
- DOI: 10.1186/s13046-019-1163-6
Resolvin D1 prevents epithelial-mesenchymal transition and reduces the stemness features of hepatocellular carcinoma by inhibiting paracrine of cancer-associated fibroblast-derived COMP
Abstract
Background: Cancer stem cells (CSCs) require stromal signals for maintaining pluripotency and self-renewal capacities to confer tumor metastasis. Resolvin D1 (RvD1), an endogenous anti-inflammatory lipid mediator, has recently been identified to display anti-cancer effects by acting on stroma cells. Our previous study reveals that hepatic stellate cells (HSCs)-derived cartilage oligomeric matrix protein (COMP) contributes to hepatocellular carcinoma (HCC) progression. However, whether RvD1 inhibits paracrine of cancer-associated fibroblasts (CAFs)-derived COMP to prevent epithelial-mesenchymal transition (EMT) and cancer stemness in HCC remains to be elucidated.
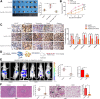
Methods: CAFs were isolated from HCC tissues. Direct and indirect co-culture models were established to analyze the interactions between HCC cells and CAFs in the presence of RvD1 in vitro. The transwell and tumor sphere formation assays were used to determine invasion and stemness of HCC cells. The subcutaneous tumor formation and orthotopic liver tumor models were established by co-implantation of CAFs and HCC cells to evaluate the role of RvD1 in vivo. To characterize the mechanism of RvD1 inhibited paracrine of COMP in CAFs, various signaling molecules were analyzed by ELISA, western blotting, reactive oxygen species (ROS) detection, immunofluorescence staining, dual luciferase reporter assay and chromatin immunoprecipitation assay.
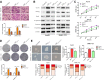
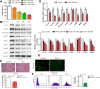
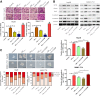
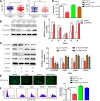
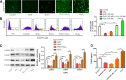
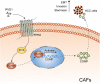
Results: Our data revealed that RvD1 treatment can impede the CAFs-induced cancer stem-like properties and the EMT of HCC cells under co-culture conditions. In vivo studies indicated that RvD1 intervention repressed the promoting effects of CAFs on tumor growth and metastasis of HCC. Furthermore, RvD1 inhibited CAF-induced EMT and stemness features of HCC cells by suppressing the secretion of COMP. Mechanistically, formyl peptide receptor 2 (FPR2) receptor mediated the suppressive effects of RvD1 on COMP and forkhead box M1 (FOXM1) expression in CAFs. Notably, RvD1 impaired CAF-derived COMP in a paracrine manner by targeting FPR2/ROS/FOXM1 signaling to ultimately abrogate FOXM1 recruitment to the COMP promoter.
Conclusion: Our results indicated that RvD1 impaired paracrine of CAFs-derived COMP by targeting FPR2/ROS/FOXM1 signaling to repress EMT and cancer stemness in HCC. Thus, RvD1 may be a potential agent to promote treatment outcomes in HCC.
Keywords: COMP; Cancer stemness; Cancer-associated fibroblasts; FOXM1; Hepatocellular carcinoma; ROS; Resolvin D1.
Conflict of interest statement
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Research Ethics Committee of The First Affiliated Hospital of Xi’an Jiaotong University and with the 1964 Helsinki declaration and its later amendments. ALL written informed consent to participate in the study was obtained from HCC patients for samples to be collected from them.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
HSCs-derived COMP drives hepatocellular carcinoma progression by activating MEK/ERK and PI3K/AKT signaling pathways.J Exp Clin Cancer Res. 2018 Sep 19;37(1):231. doi: 10.1186/s13046-018-0908-y. J Exp Clin Cancer Res. 2018. PMID: 30231922 Free PMC article.
-
Cancer-associated fibroblasts promote the stemness of CD24+ liver cells via paracrine signaling.J Mol Med (Berl). 2019 Feb;97(2):243-255. doi: 10.1007/s00109-018-1731-9. Epub 2018 Dec 18. J Mol Med (Berl). 2019. PMID: 30564864
-
FoxM1 overexpression promotes epithelial-mesenchymal transition and metastasis of hepatocellular carcinoma.World J Gastroenterol. 2015 Jan 7;21(1):196-213. doi: 10.3748/wjg.v21.i1.196. World J Gastroenterol. 2015. PMID: 25574092 Free PMC article.
-
Emerging Role of Cancer-Associated Fibroblasts in Progression and Treatment of Hepatocellular Carcinoma.Int J Mol Sci. 2023 Feb 15;24(4):3941. doi: 10.3390/ijms24043941. Int J Mol Sci. 2023. PMID: 36835352 Free PMC article. Review.
-
Exosomes in bridging macrophage-fibroblast polarity and cancer stemness.Med Oncol. 2025 May 21;42(6):216. doi: 10.1007/s12032-025-02774-6. Med Oncol. 2025. PMID: 40397051 Review.
Cited by
-
Microvesicles: the functional mediators in sorafenib resistance.Cancer Drug Resist. 2022 Jun 23;5(3):749-761. doi: 10.20517/cdr.2021.137. eCollection 2022. Cancer Drug Resist. 2022. PMID: 36176764 Free PMC article. Review.
-
Cartilage Oligomeric Matrix Protein, Diseases, and Therapeutic Opportunities.Int J Mol Sci. 2022 Aug 17;23(16):9253. doi: 10.3390/ijms23169253. Int J Mol Sci. 2022. PMID: 36012514 Free PMC article. Review.
-
Cancer Stem Cells: A Potential Breakthrough in HCC-Targeted Therapy.Front Pharmacol. 2020 Mar 6;11:198. doi: 10.3389/fphar.2020.00198. eCollection 2020. Front Pharmacol. 2020. PMID: 32210805 Free PMC article. Review.
-
Current insights into the hepatic microenvironment and advances in immunotherapy for hepatocellular carcinoma.Front Immunol. 2023 May 18;14:1188277. doi: 10.3389/fimmu.2023.1188277. eCollection 2023. Front Immunol. 2023. PMID: 37275909 Free PMC article. Review.
-
Multi-omics cluster defines the subtypes of CRC with distinct prognosis and tumor microenvironment.Eur J Med Res. 2024 Mar 28;29(1):207. doi: 10.1186/s40001-024-01805-8. Eur J Med Res. 2024. PMID: 38549156 Free PMC article.
References
-
- Tu K, Li J, Verma VK, Liu C, Billadeau DD, Lamprecht G, Xiang X, Guo L, Dhanasekaran R, Roberts LR, et al. Vasodilator-stimulated phosphoprotein promotes activation of hepatic stellate cells by regulating Rab11-dependent plasma membrane targeting of transforming growth factor beta receptors. Hepatology. 2015;61:361–374. doi: 10.1002/hep.27251. - DOI - PMC - PubMed
-
- Dou C, Liu Z, Tu K, Zhang H, Chen C, Yaqoob U, Wang Y, Wen J, van Deursen J, Sicard D, et al. P300 acetyltransferase mediates stiffness-induced activation of hepatic stellate cells into tumor-promoting Myofibroblasts. Gastroenterology. 2018;154:2209–2221 e2214. doi: 10.1053/j.gastro.2018.02.015. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

