SMS SOS: a randomized controlled trial to reduce self-harm and suicide attempts using SMS text messaging
- PMID: 30999952
- PMCID: PMC6471753
- DOI: 10.1186/s12888-019-2104-9
SMS SOS: a randomized controlled trial to reduce self-harm and suicide attempts using SMS text messaging
Abstract
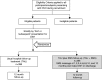
Background: Hospital-treated deliberate self-harm (DSH) is common, costly and has high repetition rates. Since brief contact interventions (BCIs) may reduce the risk of DSH repetition, we aim to evaluate whether a SMS (Short Message Service) text message Intervention plus Treatment As Usual (TAU) compared to TAU alone will reduce hospital DSH re-presentation rates in Western Sydney public hospitals in Australia.
Methods/design: Our study is a 24-month randomized controlled trial (RCT). Adult patients who present with DSH to hospital emergency, psychiatric, and mental health triage and assessment departments will be randomly assigned to an Intervention condition plus TAU receiving nine SMS text messages at 1, 2, 3, 4, 5, 6, 8, 10 and 12-months post-discharge. Each message will contain telephone numbers for two mental health crises support tele-services. Primary outcomes will be the difference in the number of DSH re-presentations, and the time to first re-presentation, within 12-months of discharge.
Discussion: This study protocol describes the design and implementation of an RCT using SMS text messages, which aim to reduce hospital re-presentation rates for DSH. Positive study findings would support the translation of an SMS-aftercare protocol into mental health services at minimal expense.
Trial registration and ethics approval: This trial has been registered with the Australian and New Zealand Clinical Trials Registry (Trial registration: ACTRN12617000607370 . Registered 28 April 2017) and has been approved by two Local Health Districts (LHDs). Western Sydney LHD Human Research Ethics Committee approved the study for Westmead Hospital and Blacktown Hospital (Protocol: HREC/16/WMEAD/336). Nepean Blue Mountains LHD Research Governance Office approved the study for Nepean Hospital (SSA/16/Nepean/170).
Keywords: Deliberate self-harm; Intentional self-harm; Prevention; Randomized controlled trial; Re-present; Reattempt; SMS; Short message service; Suicide; Text message.
Conflict of interest statement
Ethics approval and consent to participate
This trial has been registered with the Australian New Zealand Clinical Trials Registry (ACTRN12617000607370) and has been approved by the Western Sydney Local Health District Human Research Ethics Committee (Protocol: HREC/16/WMEAD/336). Site Specific Assessment was approved by Nepean Blue Mountains Local Health District Research Governance Office (SSA/16/Nepean/170). Participants in the Intervention condition will be required to provide their informed consent before receiving SMS text messages, as per the Zelen recruitment method [11].
Consent for publication
Not applicable.
Competing interests
The authors (GJS, TEH, SB, MA, AdR, JH, TC, RB, JM, JA, AK, NG, AP, GG, CJR, IMW, GLC, and AJ) declare they have no competing interests:
In the past 5 years, they didn’t receive reimbursements, fees, funding, or salaries from an organization that may in any way gain or lose financially from the publication of this manuscript.
They don’t hold any stocks or shares in an organization that may in any way gain or lose financially from the publication of this manuscript, either now or in the future.
They don’t receive reimbursements, fees, funding, or salaries from any organization that holds or has applied for patents relating to the content of the manuscript.
They don’t have any other financial or non-financial competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Carter Gregory, Page Andrew, Large Matthew, Hetrick Sarah, Milner Allison Joy, Bendit Nick, Walton Carla, Draper Brian, Hazell Philip, Fortune Sarah, Burns Jane, Patton George, Lawrence Mark, Dadd Lawrence, Robinson Jo, Christensen Helen. Royal Australian and New Zealand College of Psychiatrists clinical practice guideline for the management of deliberate self-harm. Australian & New Zealand Journal of Psychiatry. 2016;50(10):939–1000. doi: 10.1177/0004867416661039. - DOI - PubMed
-
- Australian Institute of Health and Welfare: Pointer S: Trends in hospitalised injury, Australia: 1999–00 to 2012–13. Injury research and statistics series no 95 Cat no INJCAT 171. Canberra: AIHW. 2015. https://www.aihw.gov.au/getmedia/fc940eea-91aa-47d6-ac20-94243a83a5e9/15.... Accessed 17 Mar 2019.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous