Rethinking sex determination of non-gonadal tissues
- PMID: 30999979
- PMCID: PMC7485614
- DOI: 10.1016/bs.ctdb.2019.01.003
Rethinking sex determination of non-gonadal tissues
Abstract
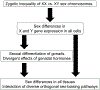
Evolution of genetic mechanisms of sex determination led to two processes causing sex differences in somatic phenotypes: gonadal differentiation and sex chromosome dosage inequality. In species with heteromorphic sex chromosomes, the sex of the individual is established at the time of formation of the zygote, leading to inherent sex differences in expression of sex chromosome genes beginning as soon as the embryonic transcriptome is activated. The inequality of sex chromosome gene expression causes sexual differentiation of the gonads and of non-gonadal tissues. The difference in gonad type in turn causes lifelong differences in gonadal hormones, which interact with unequal effects of X and Y genes acting within cells. Separating the effects of gonadal hormones and sex chromosomes has been possible using mouse models in which gonadal determination is separated from the sex chromosomes, allowing comparison of XX and XY mice with the same type of gonad. Sex differences caused by gonadal hormones and sex chromosomes affect basic physiology and disease mechanisms in most or all tissues.
Keywords: Cell-autonomous; Dosage compensation; Sex chromosomes; Sex determination; Sex difference; Sexual differentiation; X chromosome; Y chromosome.
© 2019 Elsevier Inc. All rights reserved.
Figures