Prenatal Treatment of X-Linked Hypohidrotic Ectodermal Dysplasia using Recombinant Ectodysplasin in a Canine Model
- PMID: 31000577
- PMCID: PMC6812859
- DOI: 10.1124/jpet.118.256040
Prenatal Treatment of X-Linked Hypohidrotic Ectodermal Dysplasia using Recombinant Ectodysplasin in a Canine Model
Abstract
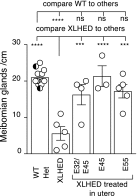
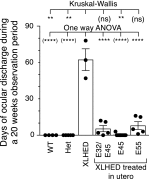
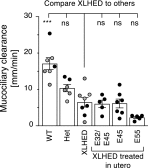
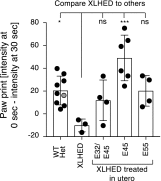
X-linked hypohidrotic ectodermal dysplasia (XLHED) is caused by defects in the EDA gene that inactivate the function of ectodysplasin A1 (EDA1). This leads to abnormal development of eccrine glands, hair follicles, and teeth, and to frequent respiratory infections. Previous studies in the naturally occurring dog model demonstrated partial prevention of the XLHED phenotype by postnatal administration of recombinant EDA1. The results suggested that a single or two temporally spaced injections of EDI200 prenatally might improve the clinical outcome in the dog model. Fetuses received ultrasound-guided EDI200 intra-amniotically at gestational days 32 and 45, or 45 or 55 alone (of a 65-day pregnancy). Growth rates, lacrimation, hair growth, meibomian glands, sweating, dentition, and mucociliary clearance were compared in treated and untreated XLHED-affected dogs, and in heterozygous and wild-type control dogs. Improved phenotypic outcomes were noted in the earlier and more frequently treated animals. All animals treated prenatally showed positive responses compared with untreated dogs with XLHED, most notably in the transfer of moisture through paw pads, suggesting improved onset of sweating ability and restored meibomian gland development. These results exemplify the feasibility of ultrasound-guided intra-amniotic injections for the treatment of developmental disorders, with improved formation of specific EDA1-dependent structures in dogs with XLHED.
Copyright © 2019 by The American Society for Pharmacology and Experimental Therapeutics.
Figures



Similar articles
-
Significant correction of disease after postnatal administration of recombinant ectodysplasin A in canine X-linked ectodermal dysplasia.Am J Hum Genet. 2007 Nov;81(5):1050-6. doi: 10.1086/521988. Epub 2007 Sep 18. Am J Hum Genet. 2007. PMID: 17924345 Free PMC article.
-
A Causal Treatment for X-Linked Hypohidrotic Ectodermal Dysplasia: Long-Term Results of Short-Term Perinatal Ectodysplasin A1 Replacement.Int J Mol Sci. 2023 Apr 12;24(8):7155. doi: 10.3390/ijms24087155. Int J Mol Sci. 2023. PMID: 37108325 Free PMC article.
-
Neonatal treatment with recombinant ectodysplasin prevents respiratory disease in dogs with X-linked ectodermal dysplasia.Am J Med Genet A. 2009 Sep;149A(9):2045-9. doi: 10.1002/ajmg.a.32916. Am J Med Genet A. 2009. PMID: 19533784 Free PMC article.
-
Future developments in XLHED treatment approaches.Am J Med Genet A. 2014 Oct;164A(10):2433-6. doi: 10.1002/ajmg.a.36499. Epub 2014 Mar 26. Am J Med Genet A. 2014. PMID: 24678015 Review.
-
A novel frameshift mutation of the EDA1 gene in a Chinese Han family with X-linked hypohidrotic ectodermal dysplasia.Clin Exp Dermatol. 2009 Jan;34(1):74-6. doi: 10.1111/j.1365-2230.2008.02844.x. Epub 2008 Aug 12. Clin Exp Dermatol. 2009. PMID: 18702659 Review.
Cited by
-
Ectodysplasin Signaling through XEDAR Is Required for Mammary Gland Morphogenesis.J Invest Dermatol. 2023 Aug;143(8):1529-1537.e2. doi: 10.1016/j.jid.2023.02.007. Epub 2023 Feb 18. J Invest Dermatol. 2023. PMID: 36804570 Free PMC article.
-
Protocol for the Phase 2 EDELIFE Trial Investigating the Efficacy and Safety of Intra-Amniotic ER004 Administration to Male Subjects with X-Linked Hypohidrotic Ectodermal Dysplasia.Genes (Basel). 2023 Jan 6;14(1):153. doi: 10.3390/genes14010153. Genes (Basel). 2023. PMID: 36672894 Free PMC article.
-
Integrated spatiotemporal transcriptomic resolution of embryonic palate osteogenesis.bioRxiv [Preprint]. 2023 Mar 30:2023.03.30.534875. doi: 10.1101/2023.03.30.534875. bioRxiv. 2023. Update in: Nat Commun. 2023 Sep 14;14(1):5687. doi: 10.1038/s41467-023-41349-9. PMID: 37333290 Free PMC article. Updated. Preprint.
-
Protein isoform-centric therapeutics: expanding targets and increasing specificity.Nat Rev Drug Discov. 2024 Oct;23(10):759-779. doi: 10.1038/s41573-024-01025-z. Epub 2024 Sep 4. Nat Rev Drug Discov. 2024. PMID: 39232238 Review.
-
Ectodysplasin A (EDA) Signaling: From Skin Appendage to Multiple Diseases.Int J Mol Sci. 2022 Aug 10;23(16):8911. doi: 10.3390/ijms23168911. Int J Mol Sci. 2022. PMID: 36012178 Free PMC article. Review.
References
-
- Abi-Nader KN, Boyd M, Flake AW, Mehta V, Peebles D, David AL. (2012) Animal models for prenatal gene therapy: the sheep model, in Methods in Molecular Biology (Coutelle C, Waddington SN. eds) pp 219–248, Humana Press, New York. - PubMed
-
- Al-Bagdadi F. (2013) The integument, in Miller’s Anatomy of the Dog (Evans HE, Miller ME, De Lahunta A. eds) pp 61–79, Elsevier, St. Louis, MO.
-
- Arita R, Fukuoka S, Morishige N. (2017) Functional morphology of the lipid layer of the tear film. Cornea 36 (Suppl 1):S60–S66. - PubMed
-
- Blüschke G, Nüsken KD, Schneider H. (2010) Prevalence and prevention of severe complications of hypohidrotic ectodermal dysplasia in infancy. Early Hum Dev 86:397–399. - PubMed
-
- Bodmer JL, Schneider P, Tschopp J. (2002) The molecular architecture of the TNF superfamily. Trends Biochem Sci 27:19–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

