Spontaneous behaviors drive multidimensional, brainwide activity
- PMID: 31000656
- PMCID: PMC6525101
- DOI: 10.1126/science.aav7893
Spontaneous behaviors drive multidimensional, brainwide activity
Abstract
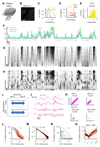
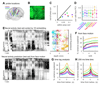
Neuronal populations in sensory cortex produce variable responses to sensory stimuli and exhibit intricate spontaneous activity even without external sensory input. Cortical variability and spontaneous activity have been variously proposed to represent random noise, recall of prior experience, or encoding of ongoing behavioral and cognitive variables. Recording more than 10,000 neurons in mouse visual cortex, we observed that spontaneous activity reliably encoded a high-dimensional latent state, which was partially related to the mouse's ongoing behavior and was represented not just in visual cortex but also across the forebrain. Sensory inputs did not interrupt this ongoing signal but added onto it a representation of external stimuli in orthogonal dimensions. Thus, visual cortical population activity, despite its apparently noisy structure, reliably encodes an orthogonal fusion of sensory and multidimensional behavioral information.
Copyright © 2019, American Association for the Advancement of Science.
Conflict of interest statement
The authors declare no competing interests.
Figures


Comment in
-
Parsing signal and noise in the brain.Science. 2019 Apr 19;364(6437):236-237. doi: 10.1126/science.aax1512. Epub 2019 Apr 18. Science. 2019. PMID: 31000652 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

