Colossal barocaloric effects near room temperature in plastic crystals of neopentylglycol
- PMID: 31000715
- PMCID: PMC6472423
- DOI: 10.1038/s41467-019-09730-9
Colossal barocaloric effects near room temperature in plastic crystals of neopentylglycol
Abstract
There is currently great interest in replacing the harmful volatile hydrofluorocarbon fluids used in refrigeration and air-conditioning with solid materials that display magnetocaloric, electrocaloric or mechanocaloric effects. However, the field-driven thermal changes in all of these caloric materials fall short with respect to their fluid counterparts. Here we show that plastic crystals of neopentylglycol (CH3)2C(CH2OH)2 display extremely large pressure-driven thermal changes near room temperature due to molecular reconfiguration, that these changes outperform those observed in any type of caloric material, and that these changes are comparable with those exploited commercially in hydrofluorocarbons. Our discovery of colossal barocaloric effects in a plastic crystal should bring barocaloric materials to the forefront of research and development in order to achieve safe environmentally friendly cooling without compromising performance.
Conflict of interest statement
The use of NPG and other plastic crystals for barocaloric cooling is covered in the following patent: X.M., A.Av., L.M., J.-Ll.T. and P.L., Use of barocaloric materials and barocaloric devices, PCT/EP2017/076203 (2017). The remaining authors declare no competing interests.
Figures

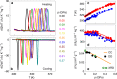
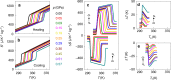

Similar articles
-
Colossal barocaloric effects in plastic crystals.Nature. 2019 Mar;567(7749):506-510. doi: 10.1038/s41586-019-1042-5. Epub 2019 Mar 27. Nature. 2019. PMID: 30918372
-
Materials with Giant Mechanocaloric Effects: Cooling by Strength.Adv Mater. 2017 Mar;29(11). doi: 10.1002/adma.201603607. Epub 2016 Dec 27. Adv Mater. 2017. PMID: 28026063 Review.
-
Understanding colossal barocaloric effects in plastic crystals.Nat Commun. 2020 Aug 21;11(1):4190. doi: 10.1038/s41467-020-18043-1. Nat Commun. 2020. PMID: 32826887 Free PMC article.
-
Giant and Reversible Inverse Barocaloric Effects near Room Temperature in Ferromagnetic MnCoGeB0.03.Adv Mater. 2019 Sep;31(37):e1903577. doi: 10.1002/adma.201903577. Epub 2019 Aug 5. Adv Mater. 2019. PMID: 31385369
-
Caloric materials near ferroic phase transitions.Nat Mater. 2014 May;13(5):439-50. doi: 10.1038/nmat3951. Nat Mater. 2014. PMID: 24751772 Review.
Cited by
-
Dynamical Disorder in the Mesophase Ferroelectric HdabcoClO4: A Machine-Learned Force Field Study.J Phys Chem C Nanomater Interfaces. 2024 Dec 18;129(1):484-494. doi: 10.1021/acs.jpcc.4c06615. eCollection 2025 Jan 9. J Phys Chem C Nanomater Interfaces. 2024. PMID: 39811432 Free PMC article.
-
Phase-dependent dielectric properties and proton conduction of neopentyl glycol.RSC Adv. 2021 Jul 5;11(38):23228-23234. doi: 10.1039/d1ra03366b. eCollection 2021 Jul 1. RSC Adv. 2021. PMID: 35479796 Free PMC article.
-
Discovery of Colossal Breathing-Caloric Effect under Low Applied Pressure in the Hybrid Organic-Inorganic MIL-53(Al) Material.Chem Mater. 2022 Apr 12;34(7):3323-3332. doi: 10.1021/acs.chemmater.2c00137. Epub 2022 Mar 30. Chem Mater. 2022. PMID: 35444364 Free PMC article.
-
Colossal barocaloric effects in the complex hydride Li[Formula: see text]B[Formula: see text]H[Formula: see text].Sci Rep. 2021 Jun 7;11(1):11915. doi: 10.1038/s41598-021-91123-4. Sci Rep. 2021. PMID: 34099742 Free PMC article.
-
Pressure-freezing of dodecane: exploring the crystal structures, formation kinetics and phase diagrams for colossal barocaloric effects in n-alkanes.RSC Adv. 2023 Nov 13;13(47):33305-33317. doi: 10.1039/d3ra06957e. eCollection 2023 Nov 7. RSC Adv. 2023. PMID: 37964902 Free PMC article.
References
-
- Tamarit JLl, Legendre B, Buisine JM. Thermodynamic study of some neopentane derivated by thermobarometric analysis. Mol. Cryst. Liq. Cryst. 1994;250:347–358. doi: 10.1080/10587259408028219. - DOI
-
- De Gennes, P. G. & Prost, J. The Physics of liquid crystals (Oxford University Press, New York, USA, 1993).
-
- Tamarit JLl, Pérez-Jubindo MA, de la Fuente MR. Dielectric studies on orientationally disordered phases of neopentylglycol ((CH3)2C(CH2OH)2) (and tris(hydroxymethyl aminomethane) ((NH2)C(CH2OH)3) J. Phys. Condens. Matter. 1997;9:5469–5478. doi: 10.1088/0953-8984/9/25/014. - DOI
-
- Timmermans J. Plastic crystals: a historical review. J. Phys. Chem. Solids. 1961;18:1–8. doi: 10.1016/0022-3697(61)90076-2. - DOI
-
- Benson DK, Burrows W, Webb JD. Solid state phase transitions in pentaerythritol and related polyhydric alcohols. Sol. Energy Mat. 1986;13:133–152. doi: 10.1016/0165-1633(86)90040-7. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

