Role of DNA Methylation in Hypobaric Hypoxia-Induced Neurodegeneration and Spatial Memory Impairment
- PMID: 31000957
- PMCID: PMC6470337
- DOI: 10.1159/000490368
Role of DNA Methylation in Hypobaric Hypoxia-Induced Neurodegeneration and Spatial Memory Impairment
Abstract
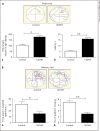
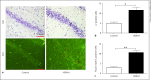
Hypobaric hypoxia (HH) is a major stress factor that is associated with physiological, biochemical, molecular and genomic alterations. Brain is the organ that reacts sensitively to oxygen deprivation, which leads to oxidative stress and cognitive function impairment. Our previous studies have reported that downregulation of brain derived neurotrophic factor (BDNF) leads to neurodegeneration and memory impairment. The aim of the present study was to investigate the effect of HH exposure on DNA methylation and its regulation in BDNF expression, neurodegeneration and spatial memory impairment. For this purpose, Sprague Dawley rats were exposed to HH at a simulated altitude of 25,000 feet for 14 days. Real-time polymerase chain reaction was used for transcriptional expression of DNA Methyltransferases (DNMTs) including DNMT1, DNMT3a and -DNMT3b, and immunoblotting was used for the translational expression of DNMT1, DNMT3a, DNMT3b, Methyl CpG binding protein 2 (MeCP2), pMeCP2 and BDNF in rat hippocampus. Additionally, neuronal morphology alteration and neurodegeneration in CA1 region of hippocampus were investigated though Cresyl violet (CV) staining and Fluoro-Jade C staining respectively. Results obtained suggested that HH exposure increased the expression of DNMT1 DNMT3b at the mRNA as well as protein level, whereas no significant change was observed in the level of DNMT3a. Furthermore, the level of pMeCP2 and BDNF were significantly decreased; however, the expression level of MeCP2 was significantly increased. The CV and Fluoro-Jade C-positive cells were significantly enhanced in the CA1 region of hippocampus in the HH exposed group as compared to unexposed rats. Thus, the present study concluded that HH decreases neuronal activation by the upregulation of DNA methylation and MeCP2 and decreased the expression of pMeCP2, which result in the downregulation of BDNF. The decreased BDNF expression is associated with neuronal loss and spatial memory impairment. This study highlights that DNMT inhibition could be an important therapeutic target for neurodegenerative diseases.
Keywords: Brain-derived neurotrophic factor; DNA methyltransferase; Hypobaric hypoxia; Methyl CpG binding protein 2; Neurodegeneration.
Figures




Similar articles
-
Trans-Himalayan Phytococktail Confers Protection Against Hypobaric Hypoxia-Induced Hippocampal Neurodegeneration and Memory Impairment in Male Sprague Dawley Rats.High Alt Med Biol. 2019 Sep;20(3):279-292. doi: 10.1089/ham.2019.0011. High Alt Med Biol. 2019. PMID: 31550185
-
Quercetin reverses hypobaric hypoxia-induced hippocampal neurodegeneration and improves memory function in the rat.High Alt Med Biol. 2013 Dec;14(4):383-94. doi: 10.1089/ham.2013.1014. High Alt Med Biol. 2013. PMID: 24377346
-
Hypobaric Hypoxia-Induced Learning and Memory Impairment: Elucidating the Role of Small Conductance Ca2+-Activated K+ Channels.Neuroscience. 2018 Sep 15;388:418-429. doi: 10.1016/j.neuroscience.2018.07.026. Epub 2018 Jul 24. Neuroscience. 2018. PMID: 30048783
-
Microbiota and memory: A symbiotic therapy to counter cognitive decline?Brain Circ. 2019 Sep 30;5(3):124-129. doi: 10.4103/bc.bc_34_19. eCollection 2019 Jul-Sep. Brain Circ. 2019. PMID: 31620659 Free PMC article. Review.
-
Mechanism, prevention and treatment of cognitive impairment caused by high altitude exposure.Front Physiol. 2023 Sep 4;14:1191058. doi: 10.3389/fphys.2023.1191058. eCollection 2023. Front Physiol. 2023. PMID: 37731540 Free PMC article. Review.
Cited by
-
The Chromatin-Oxygen Sensor Gene KDM5C Associates with Novel Hypoxia-Related Signatures in Glioblastoma Multiforme.Int J Mol Sci. 2022 Sep 6;23(18):10250. doi: 10.3390/ijms231810250. Int J Mol Sci. 2022. PMID: 36142158 Free PMC article.
-
Transcriptome Analysis and Emerging Driver Identification of CD8+ T Cells in Patients with Vitiligo.Oxid Med Cell Longev. 2019 Nov 26;2019:2503924. doi: 10.1155/2019/2503924. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31885781 Free PMC article.
-
Administration of branched-chain amino acids alters epigenetic regulatory enzymes in an animal model of Maple Syrup Urine Disease.Metab Brain Dis. 2021 Feb;36(2):247-254. doi: 10.1007/s11011-020-00631-1. Epub 2020 Oct 24. Metab Brain Dis. 2021. PMID: 33098071
-
Hypoxic Preconditioning Modulates BDNF and Its Signaling through DNA Methylation to Promote Learning and Memory in Mice.ACS Chem Neurosci. 2023 Jun 21;14(12):2320-2332. doi: 10.1021/acschemneuro.3c00069. Epub 2023 Jun 8. ACS Chem Neurosci. 2023. PMID: 37289948 Free PMC article.
-
Fetal origins of obesity: a novel pathway of regulating appetite neurons in the hypothalamus of growth-restricted rat offspring.Arch Gynecol Obstet. 2024 Jun;309(6):2411-2419. doi: 10.1007/s00404-023-07108-3. Epub 2023 Jun 28. Arch Gynecol Obstet. 2024. PMID: 37378669 Free PMC article.
References
-
- Saugy JJ, Schmitt L, Fallet S, Faiss R, Vesin JM, Bertschi M, Heinzer R, Millet GP. Sleep disordered breathing during live high-train low in normobaric versus hypobaric hypoxia. High Alt Med Biol. 2016;17:233–238. - PubMed
-
- Muthuraju S, Maiti P, Pati S, Solanki P, Sharma AK, Singh SB, Prasad D, Ilavazhagan G. Role of cholinergic markers on memory function of rats exposed to hypobaric hypoxia. Eur J Pharmacol. 2011;672:96–105. - PubMed
-
- Hota SK, Barhwal K, Ray K, Singh SB, Ilavazhagan G. Ceftriaxone rescues hippocampal neurons from excitotoxicity and enhances memory retrieval in chronic hypobaric hypoxia. Neurobiol Learn Mem. 2008;89:522–532. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

