Transcription Factors Sp8 and Sp9 Regulate Medial Ganglionic Eminence-Derived Cortical Interneuron Migration
- PMID: 31001083
- PMCID: PMC6454190
- DOI: 10.3389/fnmol.2019.00075
Transcription Factors Sp8 and Sp9 Regulate Medial Ganglionic Eminence-Derived Cortical Interneuron Migration
Abstract
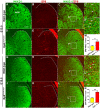
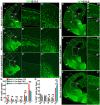
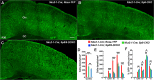
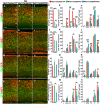
Cortical interneurons are derived from the subpallium and reach the developing cortex through long tangential migration. Mature cortical interneurons are characterized by remarkable morphological, molecular, and functional diversity. The calcium-binding protein parvalbumin (PV) and neuropeptide somatostatin (SST) identify most medial ganglionic eminence (MGE)-derived cortical interneurons. Previously, we demonstrated that Sp9 plays a curial transcriptional role in regulating MGE-derived cortical interneuron development. Here, we show that SP8 protein is weekly expressed in the MGE mantle zone of wild type mice but upregulated in Sp9 null mutants. PV+ cortical interneurons were severely lost in Sp8/Sp9 double conditional knockouts due to defects in tangential migration compared with Sp9 single mutants, suggesting that Sp8/9 coordinately regulate PV+ cortical interneuron development. We provide evidence that Sp8/Sp9 activity is required for normal MGE-derived cortical interneuron migration, at least in part, through regulating the expression of EphA3, Ppp2r2c, and Rasgef1b.
Keywords: Sp8; Sp9; cortical interneuron; medial ganglionic eminence; parvalbumin; somatostatin; tangential migration.
Figures



Similar articles
-
Sp9 Regulates Medial Ganglionic Eminence-Derived Cortical Interneuron Development.Cereb Cortex. 2019 Jun 1;29(6):2653-2667. doi: 10.1093/cercor/bhy133. Cereb Cortex. 2019. PMID: 29878134 Free PMC article.
-
Transcription factors Sp8 and Sp9 regulate the development of caudal ganglionic eminence-derived cortical interneurons.J Comp Neurol. 2019 Dec 1;527(17):2860-2874. doi: 10.1002/cne.24712. Epub 2019 May 17. J Comp Neurol. 2019. PMID: 31070778
-
Transcription Factors Sp8 and Sp9 Coordinately Regulate Olfactory Bulb Interneuron Development.Cereb Cortex. 2018 Sep 1;28(9):3278-3294. doi: 10.1093/cercor/bhx199. Cereb Cortex. 2018. PMID: 28981617 Free PMC article.
-
Transcriptional Regulation of Cortical Interneuron Development.In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 47. In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 47. PMID: 39637134 Free Books & Documents. Review.
-
Molecules and mechanisms involved in the generation and migration of cortical interneurons.ASN Neuro. 2010 Mar 31;2(2):e00031. doi: 10.1042/AN20090053. ASN Neuro. 2010. PMID: 20360946 Free PMC article. Review.
Cited by
-
Interneuron odyssey: molecular mechanisms of tangential migration.Front Neural Circuits. 2023 Sep 14;17:1256455. doi: 10.3389/fncir.2023.1256455. eCollection 2023. Front Neural Circuits. 2023. PMID: 37779671 Free PMC article. Review.
-
St18 specifies globus pallidus projection neuron identity in MGE lineage.Nat Commun. 2022 Dec 14;13(1):7735. doi: 10.1038/s41467-022-35518-5. Nat Commun. 2022. PMID: 36517477 Free PMC article.
-
SP8 Promotes an Aggressive Phenotype in Hepatoblastoma via FGF8 Activation.Cancers (Basel). 2020 Aug 15;12(8):2294. doi: 10.3390/cancers12082294. Cancers (Basel). 2020. PMID: 32824198 Free PMC article.
-
Developmental Characterization of Schizophrenia-Associated Gene Zswim6 in Mouse Forebrain.Front Neuroanat. 2021 May 13;15:669631. doi: 10.3389/fnana.2021.669631. eCollection 2021. Front Neuroanat. 2021. PMID: 34054439 Free PMC article.
-
Shh activation restores interneurons and cognitive function in newborns with intraventricular haemorrhage.Brain. 2023 Feb 13;146(2):629-644. doi: 10.1093/brain/awac271. Brain. 2023. PMID: 35867870 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

