The Role of High Mobility Group Box 1 in Ischemic Stroke
- PMID: 31001089
- PMCID: PMC6454008
- DOI: 10.3389/fncel.2019.00127
The Role of High Mobility Group Box 1 in Ischemic Stroke
Abstract
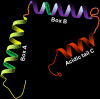
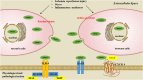
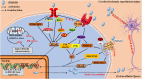
High-mobility group box 1 protein (HMGB1) is a novel, cytokine-like, and ubiquitous, highly conserved, nuclear protein that can be actively secreted by microglia or passively released by necrotic neurons. Ischemic stroke is a leading cause of death and disability worldwide, and the outcome is dependent on the amount of hypoxia-related neuronal death in the cerebral ischemic region. Acting as an endogenous danger-associated molecular pattern (DAMP) protein, HMGB1 mediates cerebral inflammation and brain injury and participates in the pathogenesis of ischemic stroke. It is thought that HMGB1 signals via its presumed receptors, such as toll-like receptors (TLRs), matrix metalloproteinase (MMP) enzymes, and receptor for advanced glycation end products (RAGEs) during ischemic stroke. In addition, the release of HMGB1 from the brain into the bloodstream influences peripheral immune cells. However, the role of HMGB1 in ischemic stroke may be more complex than this and has not yet been clarified. Here, we summarize and review the research into HMGB1 in ischemic stroke.
Keywords: high-mobility group box 1 protein (HMGB1); inflammatory response; ischemic stroke; signaling pathways; stroke-induced immunodepression.
Figures
Similar articles
-
The Roles of High Mobility Group Box 1 in Cerebral Ischemic Injury.Front Cell Neurosci. 2020 Dec 15;14:600280. doi: 10.3389/fncel.2020.600280. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33384585 Free PMC article. Review.
-
Pivotal neuroinflammatory and therapeutic role of high mobility group box 1 in ischemic stroke.Biosci Rep. 2017 Dec 5;37(6):BSR20171104. doi: 10.1042/BSR20171104. Print 2017 Dec 22. Biosci Rep. 2017. PMID: 29054968 Free PMC article. Review.
-
High-mobility group protein box-1 and its relevance to cerebral ischemia.J Cereb Blood Flow Metab. 2010 Feb;30(2):243-54. doi: 10.1038/jcbfm.2009.202. Epub 2009 Sep 30. J Cereb Blood Flow Metab. 2010. PMID: 19794402 Free PMC article. Review.
-
Review: Therapeutic Targeting of HMGB1 in Stroke.Curr Drug Deliv. 2017 Sep 6;14(6):785-790. doi: 10.2174/1567201813666160808111933. Curr Drug Deliv. 2017. PMID: 27501713 Review.
-
Enlightening the role of high mobility group box 1 (HMGB1) in inflammation: Updates on receptor signalling.Eur J Pharmacol. 2019 Sep 5;858:172487. doi: 10.1016/j.ejphar.2019.172487. Epub 2019 Jun 20. Eur J Pharmacol. 2019. PMID: 31229535 Review.
Cited by
-
Increased high-mobility group box 1 levels are associated with depression after acute ischemic stroke.Neurol Sci. 2022 May;43(5):3131-3137. doi: 10.1007/s10072-021-05571-x. Epub 2021 Nov 20. Neurol Sci. 2022. PMID: 34800198
-
The Roles of High Mobility Group Box 1 in Cerebral Ischemic Injury.Front Cell Neurosci. 2020 Dec 15;14:600280. doi: 10.3389/fncel.2020.600280. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33384585 Free PMC article. Review.
-
Translocation of High Mobility Group Box 1 From the Nucleus to the Cytoplasm in Depressed Patients With Epilepsy.ASN Neuro. 2022 Jan-Dec;14:17590914221136662. doi: 10.1177/17590914221136662. ASN Neuro. 2022. PMID: 36383501 Free PMC article.
-
Inflammatory Responses Induced by the Rupture of Intracranial Aneurysms Are Modulated by miRNAs.Mol Neurobiol. 2020 Feb;57(2):988-996. doi: 10.1007/s12035-019-01789-1. Epub 2019 Oct 25. Mol Neurobiol. 2020. PMID: 31654316 Free PMC article.
-
Inhibition of Connexin 36 attenuates HMGB1-mediated depressive-like behaviors induced by chronic unpredictable mild stress.Brain Behav. 2022 Feb;12(2):e2470. doi: 10.1002/brb3.2470. Epub 2022 Jan 28. Brain Behav. 2022. PMID: 35089644 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

