Long-Term Retention Rate of Anakinra in Adult Onset Still's Disease and Predictive Factors for Treatment Response
- PMID: 31001115
- PMCID: PMC6454864
- DOI: 10.3389/fphar.2019.00296
Long-Term Retention Rate of Anakinra in Adult Onset Still's Disease and Predictive Factors for Treatment Response
Abstract
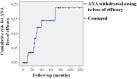
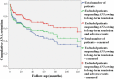
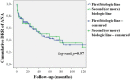
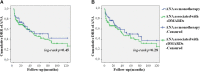
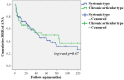
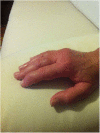
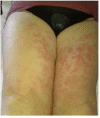
Background: Anakinra (ANA) is an effective treatment choice in patients with adult onset Still's disease (AOSD). Variables affecting treatment survival include loss of efficacy or adverse events, but also the decision to discontinue treatment after long-term clinical remission. Objectives: Aims of this study were: (i) to assess the drug retention rate (DRR) of ANA during a long-term follow-up looking for any difference related to the line of biologic treatment, the concomitant use of conventional disease modifying anti-rheumatic drugs (cDMARDs) and the different type of AOSD (systemic versus chronic articular); (ii) to identify predictive factors of lack of efficacy, loss of efficacy, and ANA withdrawal owing to long-term remission. Methods: AOSD patients classified according with Yamaguchi criteria and treated with ANA were retrospectively enrolled in 18 Italian tertiary Centers. Demographic, laboratory, clinical and therapeutic data related to the start of ANA (baseline), the 3-month assessment and the last follow-up visit while on ANA treatment were retrospectively collected and statistically analyzed. Results: One hundred and forty-one AOSD patients (48 males, 93 females) treated with ANA for a mean period of 35.96 ± 36.05 months were enrolled. The overall DRR of ANA was 44.6 and 30.5% at the 60- and 120-month assessments, respectively, with no significant differences between: (i) biologic naïve patients and those previously treated with other biologics (log-rank p = 0.97); (ii) monotherapy and concomitant use of cDMARDs (log-rank p = 0.45); (iii) systemic and chronic articular types of AOSD (log-rank p = 0.67). No variables collected at baseline could predict primary inefficacy, while the number of swollen joints at baseline was significantly associated with secondary inefficacy (p = 0.01, OR = 1.194, C.I. 1.043-1.367). The typical AOSD skin rash was negatively related with ANA withdrawal owing to long-term remission (p = 0.03, OR = 0.224, C.I. 0.058-0.863). Conclusion: Long-term DRR of ANA has been found excellent and is not affected by different lines of biologic treatment, concomitant use of cDMARDs, or type of AOSD. The risk of losing ANA efficacy increases along with the number of swollen joints at the start of therapy, while the typical skin rash is a negative predictor of ANA withdrawal related to sustained remission.
Keywords: autoinflammatory diseases; canakinumab; innovative biotechnologies; interleukin-1; personalized medicine; systemic onset juvenile idiopathic arthritis.
Figures
References
-
- Chen D. Y., Lan J. L., Lin F. J., Hsieh T. Y. (2004). Proinflammatory cytokine profiles in sera and pathological tissues of patients with active untreated adult onset Still’s disease. J. Rheumatol. 31 2189–2198. - PubMed
-
- Colafrancesco S., Priori R., Valesini G., Argolini L., Baldissera E., Bartoloni E., et al. (2017). Response to interleukin-1 inhibitors in 140 Italian patients with adult-onset still’s disease: a multicentre retrospective observational study. Front. Pharmacol. 8:369 10.3389/fphar.2017.00369 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

