Transient Receptor Potential Melastatin 8 (TRPM8)-Based Mechanisms Underlie Both the Cold Temperature-Induced Inflammatory Reactions and the Synergistic Effect of Cigarette Smoke in Human Bronchial Epithelial (16HBE) Cells
- PMID: 31001124
- PMCID: PMC6455074
- DOI: 10.3389/fphys.2019.00285
Transient Receptor Potential Melastatin 8 (TRPM8)-Based Mechanisms Underlie Both the Cold Temperature-Induced Inflammatory Reactions and the Synergistic Effect of Cigarette Smoke in Human Bronchial Epithelial (16HBE) Cells
Abstract
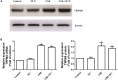
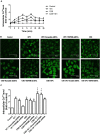
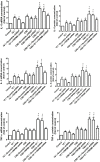
Transient receptor potential melastatin 8 (TRPM8) is a major receptor of cold environment. Recently, we found that cigarette smoke extract (CSE) upregulated TRPM8 mRNA and protein expression in bronchial tissues that made them more sensitive to cold stimuli. In our present study, we found that cold temperature (18°C)-induced activation of TRPM8 in 16HBE (human bronchial epithelial) cells facilitated Ca2+ influx and subsequently led to the increased expression of interleukin (IL)-6, IL-8, and tumor necrosis factor (TNF)-α via the upregulation of p-extracellular signal-regulated kinase (ERK) and the activation of NF-κB. In addition, 16HBE cells that co-stimulated with 18°C and CSE were used to explore the synergistic effect of CSE on cold temperature-induced inflammatory cytokine production as well as the possible involved signaling pathway. RT-PCR and western blot analysis revealed that CSE upregulated TRPM8 mRNA and protein level in 16HBE cells. Ca2+ imaging, western blot, and luciferase assay showed more robust increase in intracellular Ca2+ and promoted phosphorylated ERK, P38, and NF-κB activity, respectively, in 16HBE cells co-stimulated with CSE and cold temperature, and such alteration was attenuated by TRPM8 short hairpin RNA (shRNA) transfection and BCTC pretreatment. Furthermore, enhanced levels of IL-6, IL-8, and TNF-α showed by enzyme-linked immunosorbent assay (ELISA) were reduced by specific inhibitors of ERK and NF-κB. Collectively, our results suggest that mitogen-activated protein kinase (MAPK)/NF-κB signaling is involved in TRPM8-mediated cold temperature-induced inflammatory cytokine expression. In addition, CSE synergistically amplifies cold temperature-induced inflammatory factors release via upregulating TRPM8 expression and enhancing MAPK/NF-κB signaling pathway.
Keywords: airway inflammation; chronic obstructive pulmonary disease; cigarette smoke extract; cold temperature; transient receptor potential melastatin 8.
Figures



Similar articles
-
[Effects of transient receptor potential melastatin 8 cation channels on inflammatory reaction induced by cold temperatures in human airway epithelial cells].Zhonghua Jie He He Hu Xi Za Zhi. 2011 Oct;34(10):757-61. Zhonghua Jie He He Hu Xi Za Zhi. 2011. PMID: 22321710 Chinese.
-
Inflammatory Effects of Menthol vs. Non-menthol Cigarette Smoke Extract on Human Lung Epithelial Cells: A Double-Hit on TRPM8 by Reactive Oxygen Species and Menthol.Front Physiol. 2017 Apr 27;8:263. doi: 10.3389/fphys.2017.00263. eCollection 2017. Front Physiol. 2017. PMID: 28496415 Free PMC article.
-
Cold Stimuli Facilitate Inflammatory Responses Through Transient Receptor Potential Melastatin 8 (TRPM8) in Primary Airway Epithelial Cells of Asthmatic Mice.Inflammation. 2018 Aug;41(4):1266-1275. doi: 10.1007/s10753-018-0774-y. Inflammation. 2018. PMID: 29629494
-
Roles of TRPM8 Ion Channels in Cancer: Proliferation, Survival, and Invasion.Cancers (Basel). 2015 Oct 23;7(4):2134-46. doi: 10.3390/cancers7040882. Cancers (Basel). 2015. PMID: 26512697 Free PMC article. Review.
-
TRPM8 Puts the Chill on Prostate Cancer.Pharmaceuticals (Basel). 2016 Jul 9;9(3):44. doi: 10.3390/ph9030044. Pharmaceuticals (Basel). 2016. PMID: 27409624 Free PMC article. Review.
Cited by
-
Acute cigarette smoke or extract exposure rapidly activates TRPA1-mediated calcium influx in primary human airway smooth muscle cells.Sci Rep. 2021 May 5;11(1):9643. doi: 10.1038/s41598-021-89051-4. Sci Rep. 2021. PMID: 33953304 Free PMC article.
-
TRPA1, TRPV1, and Caffeine: Pain and Analgesia.Int J Mol Sci. 2024 Jul 19;25(14):7903. doi: 10.3390/ijms25147903. Int J Mol Sci. 2024. PMID: 39063144 Free PMC article. Review.
-
Transcriptome Analysis Revealed Inflammation Is Involved in the Impairment of Human Umbilical Vein Endothelial Cells Induced by Post-hemorrhagic Shock Mesenteric Lymph.Front Immunol. 2020 Sep 9;11:1717. doi: 10.3389/fimmu.2020.01717. eCollection 2020. Front Immunol. 2020. PMID: 33013823 Free PMC article.
-
Cold stimuli, hot topic: An updated review on the biological activity of menthol in relation to inflammation.Front Immunol. 2022 Nov 9;13:1023746. doi: 10.3389/fimmu.2022.1023746. eCollection 2022. Front Immunol. 2022. PMID: 36439160 Free PMC article. Review.
-
TRP (transient receptor potential) ion channel family: structures, biological functions and therapeutic interventions for diseases.Signal Transduct Target Ther. 2023 Jul 5;8(1):261. doi: 10.1038/s41392-023-01464-x. Signal Transduct Target Ther. 2023. PMID: 37402746 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

