Antenatal Hypoxia and Programming of Glucocorticoid Receptor Expression in the Adult Rat Heart
- PMID: 31001129
- PMCID: PMC6454194
- DOI: 10.3389/fphys.2019.00323
Antenatal Hypoxia and Programming of Glucocorticoid Receptor Expression in the Adult Rat Heart
Abstract
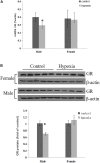
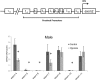
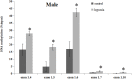
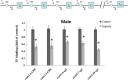
Glucocorticoid receptor (GR) signaling is critical for development and function of the heart. Our previous study demonstrated that gestational hypoxia induced epigenetic repression of the GR gene in the developing heart. The present study aims to determine that the alterations of promoter methylation level and epigenetic repression of the GR gene in the developing heart in response to maternal hypoxia is sustained in adult offspring and potential gender differences in the programming of GR gene. Pregnant rats were treated with 10.5% O2 from gestational day 15 (E15) to 21 (E21). Hearts were isolated from 5-month-old male and female offspring with the developing stage being equivalent to 18-year-old human. GR mRNA and protein abundance was determined with real time qRT-PCR and Western blot. GR gene promoter methylation and binding of transcription factors were measured with methylated DNA immunoprecipitation (MeDIP) and Chromatin immunoprecipitation (ChIP). The results showed that antenatal hypoxia significantly decreased the expression of GR mRNA and protein in the hearts of adult male offspring, but not in females, which is ascribed to the differential changes of alternative exon1 mRNA variants of GR gene in male and female hearts in response to prenatal hypoxia. In addition, the downregulation of GR expression in the male heart was correlated with increased methylation levels of CpG dinucleotides in promoters of exon 14, 15, 16, 17, and 110, which resulted in a decrease in the binding of their transcription factors. Thus, the study reveals that antenatal hypoxia results in a reprogramming and long-term change in GR gene expression in the heart by hypermethylation of GR promoter in a sex-differential pattern, which provides a novel mechanism regarding the increased vulnerability of heart later in life with exposure of prenatal hypoxia.
Keywords: DNA methylation; glucocorticoid receptor; heart; hypoxia; programming; sex.
Figures
Similar articles
-
Antenatal hypoxia induces epigenetic repression of glucocorticoid receptor and promotes ischemic-sensitive phenotype in the developing heart.J Mol Cell Cardiol. 2016 Feb;91:160-71. doi: 10.1016/j.yjmcc.2016.01.003. Epub 2016 Jan 9. J Mol Cell Cardiol. 2016. PMID: 26779948 Free PMC article.
-
Chronic prenatal hypoxia induces epigenetic programming of PKC{epsilon} gene repression in rat hearts.Circ Res. 2010 Aug 6;107(3):365-73. doi: 10.1161/CIRCRESAHA.110.221259. Epub 2010 Jun 10. Circ Res. 2010. PMID: 20538683 Free PMC article.
-
Promoter methylation of Egr-1 site contributes to fetal hypoxia-mediated PKCε gene repression in the developing heart.Am J Physiol Regul Integr Comp Physiol. 2013 May 1;304(9):R683-9. doi: 10.1152/ajpregu.00461.2012. Epub 2013 Feb 20. Am J Physiol Regul Integr Comp Physiol. 2013. PMID: 23427086 Free PMC article.
-
Foetal hypoxia impacts methylome and transcriptome in developmental programming of heart disease.Cardiovasc Res. 2019 Jul 1;115(8):1306-1319. doi: 10.1093/cvr/cvy277. Cardiovasc Res. 2019. PMID: 30395198 Free PMC article.
-
How glucocorticoid receptors modulate the activity of other transcription factors: a scope beyond tethering.Mol Cell Endocrinol. 2013 Nov 5;380(1-2):41-54. doi: 10.1016/j.mce.2012.12.014. Epub 2012 Dec 23. Mol Cell Endocrinol. 2013. PMID: 23267834 Review.
Cited by
-
Fetal hypoxia results in sex- and cell type-specific alterations in neonatal transcription in rat oligodendrocyte precursor cells, microglia, neurons, and oligodendrocytes.Cell Biosci. 2023 Mar 17;13(1):58. doi: 10.1186/s13578-023-01012-8. Cell Biosci. 2023. PMID: 36932456 Free PMC article.
-
Glucocorticoid-Dependent Mechanisms of Brain Tolerance to Hypoxia.Int J Mol Sci. 2021 Jul 26;22(15):7982. doi: 10.3390/ijms22157982. Int J Mol Sci. 2021. PMID: 34360746 Free PMC article. Review.
-
Time Domains of Hypoxia Responses and -Omics Insights.Front Physiol. 2022 Aug 8;13:885295. doi: 10.3389/fphys.2022.885295. eCollection 2022. Front Physiol. 2022. PMID: 36035495 Free PMC article. Review.
-
RETRACTED: The Emerging Role of Epigenetics in Metabolism and Endocrinology.Biology (Basel). 2023 Feb 6;12(2):256. doi: 10.3390/biology12020256. Biology (Basel). 2023. Retraction in: Biology (Basel). 2025 Jun 16;14(6):705. doi: 10.3390/biology14060705. PMID: 36829533 Free PMC article. Retracted. Review.
-
Life Course Impact of Glucocorticoids During Pregnancy on Muscle Development and Function.Front Anim Sci. 2021;2:788930. doi: 10.3389/fanim.2021.788930. Epub 2021 Dec 8. Front Anim Sci. 2021. PMID: 36325303 Free PMC article.
References
-
- Bellisario V., Panetta P., Balsevich G., Baumann V., Noble J., Raggi C., et al. (2015). Maternal high-fat diet acts as a stressor increasing maternal glucocorticoids’ signaling to the fetus and disrupting maternal behavior and brain activation in C57BL/6J mice. Psychoneuroendocrinology 60 138–150. 10.1016/j.psyneuen.2015.06.012 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

