CXCR2 Blockade Mitigates Neural Cell Injury Following Preclinical Chorioamnionitis
- PMID: 31001130
- PMCID: PMC6454349
- DOI: 10.3389/fphys.2019.00324
CXCR2 Blockade Mitigates Neural Cell Injury Following Preclinical Chorioamnionitis
Abstract
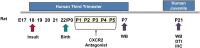
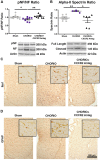
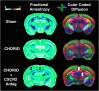
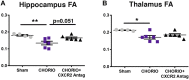
Minimizing central nervous system (CNS) injury from preterm birth depends upon identification of the critical pathways that underlie essential neurodevelopmental and CNS pathophysiology. While chorioamnionitis (CHORIO), is a leading cause of preterm birth, the precise mechanism linking prenatal brain injury and long-term CNS injury is unknown. The chemokine (C-X-C motif) ligand 1 (CXCL1) and its cognate receptor, CXCR2, are implicated in a variety of uterine and neuropathologies, however, their role in CNS injury associated with preterm birth is poorly defined. To evaluate the putative efficacy of CXCR2 blockade in neural repair secondary to CHORIO, we tested the hypothesis that transient postnatal CXCR2 antagonism would reduce neutrophil activation and mitigate cerebral microstructural injury in rats. To this end, a laparotomy was performed on embryonic day 18 (E18) in Sprague Dawley rats, with uterine arteries transiently occluded for 60 min, and lipopolysaccharide (LPS, 4 μg/sac) injected into each amniotic sac. SB225002, a CXCR2 antagonist (3 mg/kg), was administered intraperitoneally from postnatal day 1 (P1)-P5. Brains were collected on P7 and P21 and analyzed with western blot, immunohistochemistry and ex vivo diffusion tensor imaging (DTI). Results demonstrate that transient CXCR2 blockade reduced cerebral neutrophil activation (myeloperoxidase expression/MPO) and mitigated connexin43 expression, indicative of reduced neuroinflammation at P7 (p < 0.05 for all). CXCR2 blockade also reduced alpha II-spectrin calpain-mediated cleavage, improved pNF/NF ratio, and minimized Iba1 and GFAP expression consistent with improved neuronal and axonal health and reduced gliosis at P21. Importantly, DTI revealed diffuse white matter injury and decreased microstructural integrity following CHORIO as indicated by lower fractional anisotropy (FA) and elevated radial diffusivity (RD) in major white matter tracts (p < 0.05). Early postnatal CXCR2 blockade also reduced microstructural abnormalities in white matter and hippocampus at P21 (p < 0.05). Together, these data indicate that transient postnatal blockade of CXCR2 ameliorates perinatal abnormalities in inflammatory signaling, and facilitates neural repair following CHORIO. Further characterization of neuroinflammatory signaling, specifically via CXCL1/CXCR2 through the placental-fetal-brain axis, may clarify stratification of brain injury following preterm birth, and improve use of targeted interventions in this highly vulnerable patient population.
Keywords: CXCL1; alpha-II spectrin; chemokine; diffusion tensor imaging; neutrophil; preterm; white matter.
Figures


Similar articles
-
Preclinical chorioamnionitis dysregulates CXCL1/CXCR2 signaling throughout the placental-fetal-brain axis.Exp Neurol. 2018 Mar;301(Pt B):110-119. doi: 10.1016/j.expneurol.2017.11.002. Epub 2017 Nov 5. Exp Neurol. 2018. PMID: 29117499
-
CXCR2 immunomodulatory therapy protects against microstructural white matter injury and gait abnormalities but does not mitigate deficits of cognition in a preclinical model of cerebral palsy.J Neurochem. 2025 Jan;169(1):e16253. doi: 10.1111/jnc.16253. J Neurochem. 2025. PMID: 39680469 Free PMC article.
-
Sustained peripheral immune hyper-reactivity (SPIHR): an enduring biomarker of altered inflammatory responses in adult rats after perinatal brain injury.J Neuroinflammation. 2021 Oct 19;18(1):242. doi: 10.1186/s12974-021-02291-z. J Neuroinflammation. 2021. PMID: 34666799 Free PMC article.
-
The role of CXCL1/CXCR2 axis in neurological diseases.Int Immunopharmacol. 2023 Jul;120:110330. doi: 10.1016/j.intimp.2023.110330. Epub 2023 May 27. Int Immunopharmacol. 2023. PMID: 37247498 Review.
-
The role of diffusion tensor imaging and fractional anisotropy in the evaluation of patients with idiopathic normal pressure hydrocephalus: a literature review.Neurosurg Focus. 2016 Sep;41(3):E12. doi: 10.3171/2016.6.FOCUS16192. Neurosurg Focus. 2016. PMID: 27581308 Review.
Cited by
-
Peripheral immune cells and perinatal brain injury: a double-edged sword?Pediatr Res. 2022 Jan;91(2):392-403. doi: 10.1038/s41390-021-01818-7. Epub 2021 Nov 8. Pediatr Res. 2022. PMID: 34750522 Free PMC article. Review.
-
Chorioamnionitis Precipitates Perinatal Alterations of Heme-Oxygenase-1 (HO-1) Homeostasis in the Developing Rat Brain.Int J Mol Sci. 2021 May 28;22(11):5773. doi: 10.3390/ijms22115773. Int J Mol Sci. 2021. PMID: 34071287 Free PMC article.
-
IFNγ-Producing γ/δ T Cells Accumulate in the Fetal Brain Following Intrauterine Inflammation.Front Immunol. 2021 Oct 4;12:741518. doi: 10.3389/fimmu.2021.741518. eCollection 2021. Front Immunol. 2021. PMID: 34675929 Free PMC article.
-
ZIKV infection differentially affects the transcriptional profiles in HTR8 and U251 cells.Virus Res. 2023 Sep;334:199166. doi: 10.1016/j.virusres.2023.199166. Epub 2023 Jul 7. Virus Res. 2023. PMID: 37390859 Free PMC article.
-
Monocyte depletion enhances neutrophil influx and proneural to mesenchymal transition in glioblastoma.Nat Commun. 2023 Apr 3;14(1):1839. doi: 10.1038/s41467-023-37361-8. Nat Commun. 2023. PMID: 37012245 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

