Language Origins Viewed in Spontaneous and Interactive Vocal Rates of Human and Bonobo Infants
- PMID: 31001176
- PMCID: PMC6455048
- DOI: 10.3389/fpsyg.2019.00729
Language Origins Viewed in Spontaneous and Interactive Vocal Rates of Human and Bonobo Infants
Abstract
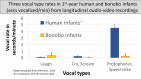
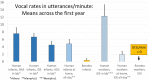
From the first months of life, human infants produce "protophones," speech-like, non-cry sounds, presumed absent, or only minimally present in other apes. But there have been no direct quantitative comparisons to support this presumption. In addition, by 2 months, human infants show sustained face-to-face interaction using protophones, a pattern thought also absent or very limited in other apes, but again, without quantitative comparison. Such comparison should provide evidence relevant to determining foundations of language, since substantially flexible vocalization, the inclination to explore vocalization, and the ability to interact socially by means of vocalization are foundations for language. Here we quantitatively compare data on vocalization rates in three captive bonobo (Pan paniscus) mother-infant pairs with various sources of data from our laboratories on human infant vocalization. Both humans and bonobos produced distress sounds (cries/screams) and laughter. The bonobo infants also produced sounds that were neither screams nor laughs and that showed acoustic similarities to the human protophones. These protophone-like sounds confirm that bonobo infants share with humans the capacity to produce vocalizations that appear foundational for language. Still, there were dramatic differences between the species in both quantity and function of the protophone and protophone-like sounds. The bonobo protophone-like sounds were far less frequent than the human protophones, and the human protophones were far less likely to be interpreted as complaints and more likely as vocal play. Moreover, we found extensive vocal interaction between human infants and mothers, but no vocal interaction in the bonobo mother-infant pairs-while bonobo mothers were physically responsive to their infants, we observed no case of a bonobo mother vocalization directed to her infant. Our cross-species comparison focuses on low- and moderate-arousal circumstances because we reason the roots of language entail vocalization not triggered by excitement, for example, during fighting or intense play. Language appears to be founded in flexible vocalization, used to regulate comfortable social interaction, to share variable affective states at various levels of arousal, and to explore vocalization itself.
Keywords: babbling; bonobo; comparative psychology; evolution of language; human evolution; infant directed speech; origin of language; parent–infant interaction.
Figures




Similar articles
-
Protophones, the precursors to speech, dominate the human infant vocal landscape.Philos Trans R Soc Lond B Biol Sci. 2021 Oct 25;376(1836):20200255. doi: 10.1098/rstb.2020.0255. Epub 2021 Sep 6. Philos Trans R Soc Lond B Biol Sci. 2021. PMID: 34482735 Free PMC article.
-
Preterm and full term infant vocalization and the origin of language.Sci Rep. 2019 Oct 14;9(1):14734. doi: 10.1038/s41598-019-51352-0. Sci Rep. 2019. PMID: 31611607 Free PMC article.
-
Perspectives on the origin of language: Infants vocalize most during independent vocal play but produce their most speech-like vocalizations during turn taking.PLoS One. 2022 Dec 30;17(12):e0279395. doi: 10.1371/journal.pone.0279395. eCollection 2022. PLoS One. 2022. PMID: 36584126 Free PMC article.
-
Temporal modulation in speech, music, and animal vocal communication: evidence of conserved function.Ann N Y Acad Sci. 2019 Oct;1453(1):99-113. doi: 10.1111/nyas.14228. Epub 2019 Sep 4. Ann N Y Acad Sci. 2019. PMID: 31482571 Review.
-
Gestural and symbolic development among apes and humans: support for a multimodal theory of language evolution.Front Psychol. 2014 Oct 30;5:1228. doi: 10.3389/fpsyg.2014.01228. eCollection 2014. Front Psychol. 2014. PMID: 25400607 Free PMC article. Review.
Cited by
-
Temporal Coordination in Mother-Infant Vocal Interaction: A Cross-Cultural Comparison.Front Psychol. 2019 Nov 8;10:2374. doi: 10.3389/fpsyg.2019.02374. eCollection 2019. Front Psychol. 2019. PMID: 31780979 Free PMC article.
-
The function and evolution of child-directed communication.PLoS Biol. 2022 May 6;20(5):e3001630. doi: 10.1371/journal.pbio.3001630. eCollection 2022 May. PLoS Biol. 2022. PMID: 35522717 Free PMC article.
-
Acoustic Correlates and Adult Perceptions of Distress in Infant Speech-Like Vocalizations and Cries.Front Psychol. 2019 May 29;10:1154. doi: 10.3389/fpsyg.2019.01154. eCollection 2019. Front Psychol. 2019. PMID: 31191389 Free PMC article.
-
Testosterone, oxytocin and co-operation: A hypothesis for the origin and function of music.Front Psychol. 2023 Feb 13;14:1055827. doi: 10.3389/fpsyg.2023.1055827. eCollection 2023. Front Psychol. 2023. PMID: 36860786 Free PMC article.
-
Aping Language: Historical Perspectives on the Quest for Semantics, Syntax, and Other Rarefied Properties of Human Language in the Communication of Primates and Other Animals.Front Psychol. 2021 Jul 23;12:675172. doi: 10.3389/fpsyg.2021.675172. eCollection 2021. Front Psychol. 2021. PMID: 34366994 Free PMC article. Review.
References
-
- Belardi K. M., Watson L. R., Faldowski R., Baranek G. T., Crais B., Patten E., et al. (2017). A retrospective video analysis of canonical babbling and volubility in infants with fragile X Syndrome at 9 -12 Months of Age. J. Autism Dev. Disabil. 47 1193–1206. 10.1007/s10803-017-3033-4 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

