Structural and Functional Characterization of the Type Three Secretion System (T3SS) Needle of Pseudomonas aeruginosa
- PMID: 31001211
- PMCID: PMC6455054
- DOI: 10.3389/fmicb.2019.00573
Structural and Functional Characterization of the Type Three Secretion System (T3SS) Needle of Pseudomonas aeruginosa
Abstract
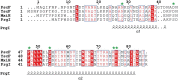
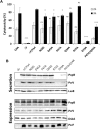
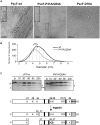
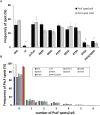
The type three secretion system (T3SS) is a macromolecular protein nano-syringe used by different bacterial pathogens to inject effectors into host cells. The extracellular part of the syringe is a needle-like filament formed by the polymerization of a 9-kDa protein whose structure and proper localization on the bacterial surface are key determinants for efficient toxin injection. Here, we combined in vivo, in vitro, and in silico approaches to characterize the Pseudomonas aeruginosa T3SS needle and its major component PscF. Using a combination of mutagenesis, phenotypic analyses, immunofluorescence, proteolysis, mass spectrometry, atomic force microscopy, electron microscopy, and molecular modeling, we propose a model of the P. aeruginosa needle that exposes the N-terminal region of each PscF monomer toward the outside of the filament, while the core of the fiber is formed by the C-terminal helix. Among mutations introduced into the needle protein PscF, D76A, and P47A/Q54A caused a defect in the assembly of the needle on the bacterial surface, although the double mutant was still cytotoxic on macrophages in a T3SS-dependent manner and formed filamentous structures in vitro. These results suggest that the T3SS needle of P. aeruginosa displays an architecture that is similar to that of other bacterial needles studied to date and highlight the fact that small, targeted perturbations in needle assembly can inhibit T3SS function. Therefore, the T3SS needle represents an excellent drug target for small molecules acting as virulence blockers that could disrupt pathogenesis of a broad range of bacteria.
Keywords: Pseudomonas aeruginosa; T3SS needle; immunofluorescence microscopy; mutagenesis; structure; type III secretion system; virulence.
Figures



Similar articles
-
A Structure-Function-Inhibition Analysis of the Pseudomonas aeruginosa Type III Secretion Needle Protein PscF.J Bacteriol. 2020 Aug 25;202(18):e00055-20. doi: 10.1128/JB.00055-20. Print 2020 Aug 25. J Bacteriol. 2020. PMID: 32601072 Free PMC article.
-
Mutations in the Pseudomonas aeruginosa needle protein gene pscF confer resistance to phenoxyacetamide inhibitors of the type III secretion system.Antimicrob Agents Chemother. 2014;58(4):2211-20. doi: 10.1128/AAC.02795-13. Epub 2014 Jan 27. Antimicrob Agents Chemother. 2014. PMID: 24468789 Free PMC article.
-
Structure of the heterotrimeric complex that regulates type III secretion needle formation.Proc Natl Acad Sci U S A. 2007 May 8;104(19):7803-8. doi: 10.1073/pnas.0610098104. Epub 2007 Apr 30. Proc Natl Acad Sci U S A. 2007. PMID: 17470796 Free PMC article.
-
Structures of Type III Secretion System Needle Filaments.Curr Top Microbiol Immunol. 2020;427:109-131. doi: 10.1007/82_2019_192. Curr Top Microbiol Immunol. 2020. PMID: 31974760 Review.
-
Biophysical Mechanism of Protein Export by Bacterial Type III Secretion System.Chem Pharm Bull (Tokyo). 2019;67(4):341-344. doi: 10.1248/cpb.c18-00947. Chem Pharm Bull (Tokyo). 2019. PMID: 30930438 Review.
Cited by
-
Solid-State NMR Investigations of Extracellular Matrixes and Cell Walls of Algae, Bacteria, Fungi, and Plants.Chem Rev. 2022 May 25;122(10):10036-10086. doi: 10.1021/acs.chemrev.1c00669. Epub 2021 Dec 8. Chem Rev. 2022. PMID: 34878762 Free PMC article. Review.
-
RetS-mediated environmental sensing coordinates TetR-dependent regulation of type III secretion system and virulence in Pseudomonas syringae pv. actinidiae.Appl Environ Microbiol. 2025 Jul 23;91(7):e0049425. doi: 10.1128/aem.00494-25. Epub 2025 Jun 10. Appl Environ Microbiol. 2025. PMID: 40492733 Free PMC article.
-
Genetic Diversity of Type 3 Secretion System in Burkholderia s.l. and Links With Plant Host Adaptation.Front Microbiol. 2021 Oct 20;12:761215. doi: 10.3389/fmicb.2021.761215. eCollection 2021. Front Microbiol. 2021. PMID: 34745070 Free PMC article.
-
Endemic High-Risk Clone ST277 Is Related to the Spread of SPM-1-Producing Pseudomonas aeruginosa during the COVID-19 Pandemic Period in Northern Brazil.Microorganisms. 2023 Aug 11;11(8):2069. doi: 10.3390/microorganisms11082069. Microorganisms. 2023. PMID: 37630629 Free PMC article.
-
Spatial transcriptomics of planktonic and sessile bacterial populations at single-cell resolution.Science. 2021 Aug 13;373(6556):eabi4882. doi: 10.1126/science.abi4882. Science. 2021. PMID: 34385369 Free PMC article.
References
-
- Bergeron J. R. C., Fernández L., Wasney G. A., Vuckovic M., Reffuveille F., Hancock R. E. W., et al. (2016). The structure of a type 3 secretion system (T3SS) ruler protein suggests a molecular mechanism for needle length sensing. J. Biol. Chem. 291 1676–1691. 10.1074/jbc.M115.684423 - DOI - PMC - PubMed
-
- Berube B. J., Murphy K. R., Torhan M. C., Bowlin N. O., Williams J. D., Bowlin T. L., et al. (2017). Impact of type III secretion effectors and of phenoxyacetamide inhibitors of type III secretion on abscess formation in a mouse model of Pseudomonas aeruginosa infection. Antimicrob. Agents Chemother. 61 e1202–e1217. 10.1128/AAC.01202-17 - DOI - PMC - PubMed
-
- Bowlin N. O., Williams J. D., Knoten C. A., Torhan M. C., Tashjian T. F., Li B., et al. (2014). Mutations in the Pseudomonas aeruginosa needle protein gene pscF confer resistance to phenoxyacetamide inhibitors of the type III secretion system. Antimicrob. Agents Chemother. 58 2211–2220. 10.1128/AAC.02795-13 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

