Mycobacterium avium Infection in a C3HeB/FeJ Mouse Model
- PMID: 31001241
- PMCID: PMC6456659
- DOI: 10.3389/fmicb.2019.00693
Mycobacterium avium Infection in a C3HeB/FeJ Mouse Model
Abstract
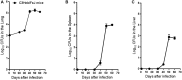
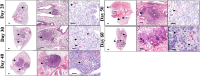
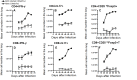
Infections caused by Mycobacterium avium complex (MAC) species are increasing worldwide, resulting in a serious public health problem. Patients with MAC lung disease face an arduous journey of a prolonged multidrug regimen that is often poorly tolerated and associated with relatively poor outcome. Identification of new animal models that demonstrate a similar pulmonary pathology as humans infected with MAC has the potential to significantly advance our understanding of nontuberculosis mycobacteria (NTM) pathogenesis as well as provide a tractable model for screening candidate compounds for therapy. One new mouse model is the C3HeB/FeJ which is similar to MAC patients in that these mice can form foci of necrosis in granulomas. In this study, we evaluated the ability of C3HeB/FeJ mice exposure to an aerosol infection of a rough strain of MAC 2285 to produce a progressive infection resulting in small necrotic foci during granuloma formation. C3HeB/FeJ mice were infected with MAC and demonstrated a progressive lung infection resulting in an increase in bacterial burden peaking around day 40, developed micronecrosis in granulomas and was associated with increased influx of CD4+ Th1, Th17, and Treg lymphocytes into the lungs. However, during chronic infection around day 50, the bacterial burden plateaued and was associated with the reduced influx of CD4+ Th1, Th17 cells, and increased numbers of Treg lymphocytes and necrotic foci during granuloma formation. These results suggest the C3HeB/FeJ MAC infection mouse model will be an important model to evaluate immune pathogenesis and compound efficacy.
Keywords: C3HeB/FeJ mouse model; Mycobacterium avium; immunity; nontuberculosis mycobacteria; pathology.
Figures


Similar articles
-
C3HeB/FeJ mice with chronic Mycobacterium avium complex pulmonary infection exhibit impaired respiratory function but not necrotising granulomatous disease.Mycobacteria. 2025;1(1):4. doi: 10.1186/s44350-025-00004-7. Epub 2025 May 15. Mycobacteria. 2025. PMID: 40386578 Free PMC article.
-
Pulmonary granuloma formation during latent Cryptococcus neoformans infection in C3HeB/FeJ mice involves progression through three immunological phases.mBio. 2025 Feb 5;16(2):e0361024. doi: 10.1128/mbio.03610-24. Epub 2025 Jan 14. mBio. 2025. PMID: 39807873 Free PMC article.
-
Differential Mycobacterium bovis BCG vaccine-derived efficacy in C3Heb/FeJ and C3H/HeOuJ mice exposed to a clinical strain of Mycobacterium tuberculosis.Clin Vaccine Immunol. 2015 Jan;22(1):91-8. doi: 10.1128/CVI.00466-14. Epub 2014 Nov 12. Clin Vaccine Immunol. 2015. PMID: 25392011 Free PMC article.
-
Mycobacterium avium complex and other nontuberculous mycobacteria in patients with HIV infection.Semin Respir Infect. 1989 Jun;4(2):123-32. Semin Respir Infect. 1989. PMID: 2664936 Review.
-
Infection Sources of a Common Non-tuberculous Mycobacterial Pathogen, Mycobacterium avium Complex.Front Med (Lausanne). 2017 Mar 7;4:27. doi: 10.3389/fmed.2017.00027. eCollection 2017. Front Med (Lausanne). 2017. PMID: 28326308 Free PMC article. Review.
Cited by
-
Additive Effects of Cyclic Peptide [R4W4] When Added Alongside Azithromycin and Rifampicin against Mycobacterium avium Infection.Pathogens. 2023 Aug 18;12(8):1057. doi: 10.3390/pathogens12081057. Pathogens. 2023. PMID: 37624017 Free PMC article.
-
Protein-energy restriction-induced lipid metabolism disruption causes stable-to-progressive disease shift in Mycobacterium avium-infected female mice.EBioMedicine. 2024 Jul;105:105198. doi: 10.1016/j.ebiom.2024.105198. Epub 2024 Jun 17. EBioMedicine. 2024. PMID: 38889480 Free PMC article.
-
Non-Invasive Mycobacterium avium Detection Using 99mTc-GSA on Single-Photon Emission Computed Tomography.Pharmaceutics. 2025 Mar 13;17(3):362. doi: 10.3390/pharmaceutics17030362. Pharmaceutics. 2025. PMID: 40143026 Free PMC article.
-
Preclinical murine models for the testing of antimicrobials against Mycobacterium abscessus pulmonary infections: Current practices and recommendations.Tuberculosis (Edinb). 2024 Jul;147:102503. doi: 10.1016/j.tube.2024.102503. Epub 2024 Mar 19. Tuberculosis (Edinb). 2024. PMID: 38729070 Free PMC article. Review.
-
The Many Hosts of Mycobacteria 8 (MHM8): A conference report.Tuberculosis (Edinb). 2020 Mar;121:101914. doi: 10.1016/j.tube.2020.101914. Epub 2020 Feb 11. Tuberculosis (Edinb). 2020. PMID: 32279870 Free PMC article. Review.
References
-
- Abendano N., Tyukalova L., Barandika J. F., Balseiro A., Sevilla I. A., Garrido J. M., et al. (2014). Mycobacterium Avium subsp. paratuberculosis isolates induce in vitro granuloma formation and show successful survival phenotype, common anti-inflammatory and antiapoptotic responses within ovine macrophages regardless of genotype or host of origin. PLoS One 9:e104238. 10.1371/journal.pone.0104238 - DOI - PMC - PubMed
-
- Blanchard J. D., Elias V., Cipolla D., Gonda I., Bermudez L. E. (2018). Effective Treatment of Mycobacterium avium subsp. hominissuis and Mycobacterium abscessus species infections in macrophages, biofilm, and mice by using liposomal ciprofloxacin. Antimicrob. Agents Chemother. 62:e00440-18. 10.1128/AAC.00440-18 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

