Dual Role of CD4 in Peripheral T Lymphocytes
- PMID: 31001252
- PMCID: PMC6454155
- DOI: 10.3389/fimmu.2019.00618
Dual Role of CD4 in Peripheral T Lymphocytes
Abstract
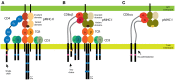
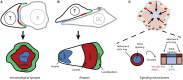
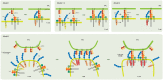
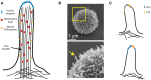
The interaction of T-cell receptors (TCRs) with self- and non-self-peptides in the major histocompatibility complex (MHC) stimulates crucial signaling events, which in turn can activate T lymphocytes. A variety of accessory molecules further modulate T-cell signaling. Of these, the CD4 and CD8 coreceptors make the most critical contributions to T cell sensitivity in vivo. Whereas, CD4 function in T cell development is well-characterized, its role in peripheral T cells remains incompletely understood. It was originally suggested that CD4 stabilizes weak interactions between TCRs and peptides in the MHC and delivers Lck kinases to that complex. The results of numerous experiments support the latter role, indicating that the CD4-Lck complex accelerates TCR-triggered signaling and controls the availability of the kinase for TCR in the absence of the ligand. On the other hand, extremely low affinity of CD4 for MHC rules out its ability to stabilize the receptor-ligand complex. In this review, we summarize the current knowledge on CD4 in T cells, with a special emphasis on the spatio-temporal organization of early signaling events and the relevance for CD4 function. We further highlight the capacity of CD4 to interact with the MHC in the absence of TCR. It drives the adhesion of T cells to the cells that express the MHC. This process is facilitated by the CD4 accumulation in the tips of microvilli on the surface of unstimulated T cells. Based on these observations, we suggest an alternative model of CD4 role in T-cell activation.
Keywords: CD4; Lck; T lymphocytes; TCR coreceptor; cell-cell adhesion; microvilli.
Figures
Similar articles
-
Subcellular distribution of Lck during CD4 T-cell maturation in the thymic medulla regulates the T-cell activation threshold.Proc Natl Acad Sci U S A. 2012 May 8;109(19):7415-20. doi: 10.1073/pnas.1119272109. Epub 2012 Apr 23. Proc Natl Acad Sci U S A. 2012. PMID: 22529380 Free PMC article.
-
[Coreceptor function of CD4 in response to MHC class I molecule].Mol Biol (Mosk). 2008 Jul-Aug;42(4):662-72. Mol Biol (Mosk). 2008. PMID: 18856067 Russian.
-
Two receptors, two kinases, and T cell lineage determination.Sci Signal. 2010 Mar 23;3(114):pe11. doi: 10.1126/scisignal.3114pe11. Sci Signal. 2010. PMID: 20332426 Review.
-
Constitutively active Lck kinase in T cells drives antigen receptor signal transduction.Immunity. 2010 Jun 25;32(6):766-77. doi: 10.1016/j.immuni.2010.05.011. Epub 2010 Jun 11. Immunity. 2010. PMID: 20541955 Free PMC article.
-
The roles of CD4 and CD8 in T cell activation.Semin Immunol. 1991 May;3(3):133-41. Semin Immunol. 1991. PMID: 1909592 Review.
Cited by
-
Suppression of Dynamical Network Biomarker Signals at the Predisease State (Mibyou) before Metabolic Syndrome in Mice by a Traditional Japanese Medicine (Kampo Formula) Bofutsushosan.Evid Based Complement Alternat Med. 2020 Aug 4;2020:9129134. doi: 10.1155/2020/9129134. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 32831883 Free PMC article.
-
A review of CD4+ T cell differentiation and diversity in dogs.Vet Immunol Immunopathol. 2024 Sep;275:110816. doi: 10.1016/j.vetimm.2024.110816. Epub 2024 Aug 21. Vet Immunol Immunopathol. 2024. PMID: 39173398 Review.
-
Peripheral CD4 +CD8 + double positive T cells: A potential marker to evaluate renal impairment susceptibility during systemic lupus erythematosus.J Biomed Res. 2022 Sep 28;37(1):59-68. doi: 10.7555/JBR.36.20220094. Online ahead of print. J Biomed Res. 2022. PMID: 36625011 Free PMC article.
-
Preparation and characterization of monoclonal antibodies recognizing two CD4 isotypes of Microminipigs.PLoS One. 2020 Nov 25;15(11):e0242572. doi: 10.1371/journal.pone.0242572. eCollection 2020. PLoS One. 2020. PMID: 33237936 Free PMC article.
-
Closing the Door with CRISPR: Genome Editing of CCR5 and CXCR4 as a Potential Curative Solution for HIV.BioTech (Basel). 2022 Jul 14;11(3):25. doi: 10.3390/biotech11030025. BioTech (Basel). 2022. PMID: 35892930 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

