PD-1 Primarily Targets TCR Signal in the Inhibition of Functional T Cell Activation
- PMID: 31001256
- PMCID: PMC6455061
- DOI: 10.3389/fimmu.2019.00630
PD-1 Primarily Targets TCR Signal in the Inhibition of Functional T Cell Activation
Abstract
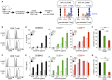
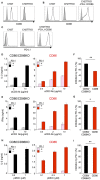
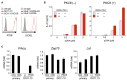
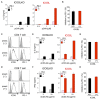
Cancer-immunotherapy targeting programmed cell death 1 (PD-1) activates tumor-specific T cells and provides clinical benefits in various cancers. However, the molecular basis of PD-1 function is still enigmatic. Especially, it is unclear which signaling pathway PD-1 primarily targets. Besides, the capacity of PD-1 to inhibit the T cell receptor (TCR)-dependent activation of T cells in the presence of co-stimulation is also controversial. Here we used co-culture systems of T cells and antigen-presenting cells with targeted deletion and overexpression of co-receptors and ligands and examined the inhibitory potency of PD-1 against T cell activation upon TCR stimulation with CD28 and ICOS co-stimulation. As an unambiguous criterion of T cell activation, we used the acquisition of cytokine production capacity, which represents one of the most important functions of T cells. PD-1 inhibited functional T cell activation upon TCR stimulation in the absence as well as in the presence of CD28 co-stimulation, indicating that PD-1 can directly inhibit TCR signal. Notably, CD28 co-stimulation rather attenuated the efficiency of PD-1 in inhibiting TCR-dependent functional T cell activation. In addition, PD-1 inhibited TCR-dependent functional T cell activation with ICOS co-stimulation as efficiently as that with CD28 co-stimulation. Furthermore, we found that the maintenance of antigen-induced follicular helper T (TFH) cells that required ICOS co-stimulation was persistently restrained by PD-1 in vivo. These findings indicate that PD-1 primarily targets TCR signal in the inhibition of functional T cell activation. Thus, PD-1 functions as the rheostat of T cell activation rather than an inhibitor of a specific stimulatory co-receptor.
Keywords: CD28; ICOS; PD-1; T cell receptor; co-receptor; cytokine production; follicular helper T cell.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

