Medroxyprogesterone Acetate Decreases Th1, Th17, and Increases Th22 Responses via AHR Signaling Which Could Affect Susceptibility to Infections and Inflammatory Disease
- PMID: 31001262
- PMCID: PMC6456711
- DOI: 10.3389/fimmu.2019.00642
Medroxyprogesterone Acetate Decreases Th1, Th17, and Increases Th22 Responses via AHR Signaling Which Could Affect Susceptibility to Infections and Inflammatory Disease
Abstract
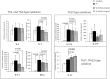
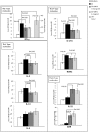
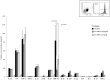
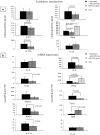
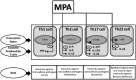
A synthetic progestin, medroxyprogesterone acetate (MPA), was used in a novel study to determine progestin effects on human purified macrophages and Th1, Th2, Th17, Th22 cells. MPA concentrations were equivalent to those in the serum of women after 6 and 9 months of progestin use. MPA has no effect on the proliferation of PBMCs and CD4+ T cell clones induced by immobilized anti-CD3 antibodies or by antigen (streptokinase). However, MPA decreases production and mRNA expression of IL-5, IL-13, IFN-γ, T-bet, RORC, and IL-17A but increases production and mRNA expression of IL-22 by CD4+ Th22 cell clones and decreases IL-22 production by Th17 cells. MPA inhibits RORC, but not T-bet and AHR, by Th17 cells but increases AHR mRNA and T-bet expression of established CD4+ Th22 cell clones. This suggests that MPA, at concentrations equivalent to those found in the serum of women after treatment for contraception and hormone replacement therapy, can directly inhibit Th1 responses (against intracellular bacteria and viruses), Th17 (against extracellular bacteria and fungi), Th2 (against parasites) but MPA therapy increases IL-22 produced by Th22 cells mediated by an increased expression of AHR and T-bet controlling inflammation. MPA could be responsible for the tissue damage limited by IL-22 in absence of IL-17A.
Keywords: Th1; Th17; Th2; Th22; contraception; hormone replacement therapy; infection; medroxyprogesterone acetate.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

