Measurement of Typhim Vi IgG as a Diagnostic Tool to Determine Anti-polysaccharide Antibody Production Deficiency in Children
- PMID: 31001267
- PMCID: PMC6455213
- DOI: 10.3389/fimmu.2019.00654
Measurement of Typhim Vi IgG as a Diagnostic Tool to Determine Anti-polysaccharide Antibody Production Deficiency in Children
Abstract
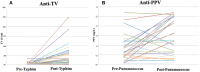
Background: The assessment of specific polysaccharide antibody production plays a pivotal role in the diagnosis of humoral primary immunodeficiencies (PID). The response to 23-valent pneumococcal vaccine (PPV) remains the gold standard for the diagnosis of polysaccharide antibodies. However, in Spain, the interpretation of pure polysaccharide 23-valent immunization is hampered by the high endemicity of pneumococcal disease and the generalization of the 13-valent adjuvant pneumococcal vaccination. Specific Typhim Vi vaccination (TV) immunoglobulin G IgG response to immunization is useful in adult PID, but there is no data regarding children. Objectives: To evaluate the clinical utility of TV IgG production as a diagnostic tool to determine anti-polysaccharide antibody production deficiency in children, when the response to PPV is unclear and isolated determination of serotypes is unfeasible. Methods: We conducted a single-institution prospective observational study on 61 children with recurrent infections. Baseline specific antibodies against PPV and TV were evaluated. In 28 children (46%), the response to the production of antibodies confirmed a clinical suspicion of humoral PID, and they were therefore immunized with 23-valent pneumococcal vaccine and Typhim Vi. Both specific antibody responses were measured by ELISA (The Binding Site Group Ltd, Birmingham, UK) using previously published cut-offs. Results: Seventy percent of the 61 children displayed baseline PPV IgG > 27 mg/L, whereas only 8% showed TV IgG > 28 U/mL (p < 0.0001). Twenty-one of 28 children (75%) achieved a 3-fold increase in post-vaccination TV IgG levels, whereas only 3% achieved a 4-fold increase in PPV IgG post vaccination, mainly due to high baseline PPV IgG titers. When we classified children according to their response to TV as responders or non-responders and compared this with the well-known clinical warning signs of the Jeffrey Modell Foundation. The proportions of children with history of pneumonia and the need for intravenous antibiotics were significantly higher in TV IgG non-responders than in TV IgG responders (p = 0.02 and p = 0.01, respectively). Conclusion: Response to TV can be considered an ancillary diagnostic tool to determine polysaccharide antibodies in children, particularly when isolated determination of pneumococcal serotypes is not feasible. TV provides a useful asset for clinicians in the era of conjugate PPV vaccination, with clinical relevance. Further research is warranted for validation.
Keywords: Typhim Vi; antibody deficiencies; children; immunodefiency; polyssaccharide; vaccine.
Figures
Similar articles
-
The Use of Salmonella Typhim Vaccine to Diagnose Antibody Deficiency.J Clin Immunol. 2017 Jul;37(5):427-433. doi: 10.1007/s10875-017-0406-6. Epub 2017 Jun 7. J Clin Immunol. 2017. PMID: 28589420
-
Defining Polysaccharide Antibody Deficiency: Measurement of Anti-Pneumococcal Antibodies and Anti-Salmonella typhi Antibodies in a Cohort of Patients with Recurrent Infections.J Clin Immunol. 2020 Jan;40(1):105-113. doi: 10.1007/s10875-019-00691-8. Epub 2019 Nov 8. J Clin Immunol. 2020. PMID: 31705452
-
Measurement of Typhi Vi antibodies can be used to assess adaptive immunity in patients with immunodeficiency.Clin Exp Immunol. 2018 Jun;192(3):292-301. doi: 10.1111/cei.13105. Epub 2018 Apr 1. Clin Exp Immunol. 2018. PMID: 29377063 Free PMC article.
-
Measurement and interpretation of Salmonella typhi Vi IgG antibodies for the assessment of adaptive immunity.J Immunol Methods. 2018 Aug;459:1-10. doi: 10.1016/j.jim.2018.05.013. Epub 2018 May 22. J Immunol Methods. 2018. PMID: 29800575 Review.
-
Immunogenicity differences of a 15-valent pneumococcal polysaccharide conjugate vaccine (PCV15) based on vaccine dose, route of immunization and mouse strain.Vaccine. 2017 Feb 7;35(6):865-872. doi: 10.1016/j.vaccine.2016.12.055. Epub 2017 Jan 10. Vaccine. 2017. PMID: 28087148 Review.
Cited by
-
Focusing on Good Responders to Pneumococcal Polysaccharide Vaccination in General Hospital Patients Suspected for Immunodeficiency. A Decision Tree Based on the 23-Valent Pneumococcal IgG Assay.Front Immunol. 2019 Nov 5;10:2496. doi: 10.3389/fimmu.2019.02496. eCollection 2019. Front Immunol. 2019. PMID: 31749801 Free PMC article.
-
The Immune Response to SARS-CoV-2 Vaccination: Insights Learned From Adult Patients With Common Variable Immune Deficiency.Front Immunol. 2022 Jan 19;12:815404. doi: 10.3389/fimmu.2021.815404. eCollection 2021. Front Immunol. 2022. PMID: 35126372 Free PMC article.
-
Variable immunodeficiency score upfront analytical link (VISUAL), a proposal for combined prognostic score at diagnosis of common variable immunodeficiency.Sci Rep. 2021 Jun 9;11(1):12211. doi: 10.1038/s41598-021-91791-2. Sci Rep. 2021. PMID: 34108596 Free PMC article.
-
Functional CVIDs phenotype clusters identified by the integration of immune parameters after BNT162b2 boosters.Front Immunol. 2023 May 25;14:1194225. doi: 10.3389/fimmu.2023.1194225. eCollection 2023. Front Immunol. 2023. PMID: 37304298 Free PMC article.
-
Vaccination in PADs.Vaccines (Basel). 2021 Jun 9;9(6):626. doi: 10.3390/vaccines9060626. Vaccines (Basel). 2021. PMID: 34207916 Free PMC article. Review.
References
-
- Sánchez-Ramón S, de Gracia J, García-Alonso AM, Rodríguez Molina JJ, Melero J, de Andrés A, et al. . Multicenter study for the evaluation of the antibody response against salmonella typhi Vi vaccination (EMPATHY) for the diagnosis of Anti-polysaccharide antibody production deficiency in patients with primary immunodeficiency. Clin Immunol. (2016) 169:80–4. 10.1016/j.clim.2016.05.006 - DOI - PubMed
-
- Orange JS, Ballow M, Stiehm ER, Ballas ZK, Chinen J, De La Morena M, et al. . Use and interpretation of diagnostic vaccination in primary immunodeficiency: a working group report of the Basic and Clinical Immunology Interest Section of the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. (2012) 130:S1–24. 10.1016/j.jaci.2012.07.002 - DOI - PubMed
-
- WHO SAGE Assessment Reports. WHO. (2018). Available online at: http://www.who.int/immunization/global_vaccine_action_plan/sage_assessme... (Accessed July 2, 2018).
-
- ACIP Vaccine Recommendations. CDC. (2018). Available online at: https://www.cdc.gov/vaccines/hcp/acip-recs/index.html (Accessed July 2, 2018).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical