Oral Administration of Compound Probiotics Improved Canine Feed Intake, Weight Gain, Immunity and Intestinal Microbiota
- PMID: 31001271
- PMCID: PMC6454072
- DOI: 10.3389/fimmu.2019.00666
Oral Administration of Compound Probiotics Improved Canine Feed Intake, Weight Gain, Immunity and Intestinal Microbiota
Abstract
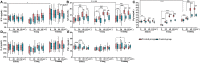
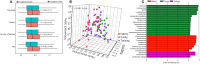
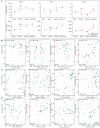
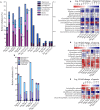
Probiotics have been used successfully to promote human and animal health, but only limited studies have focused on using probiotics to improve the health of hosts of different age. Canine microbiome studies may be predictive of results in humans because of the high structural and functional similarity between dog and human microbiomes. A total of 90 dogs were divided into three groups based on dog age (elderly group, n = 30; young group, n = 24; and training group, n = 36). Each group was subdivided into two subgroups, with and without receiving daily probiotic feed additive. The probiotic feed additive contained three different bacterial strains, namely Lactobacillus casei Zhang, Lactobacillus plantarum P-8, and Bifdobacterium animalis subsp. lactis V9. Serum and fecal samples were collected and analyzed at four different time points, i.e., days 0, 30, and 60 of probiotic treatment, and 15 days after ceasing probiotic treatment. The results demonstrated that probiotics significantly promoted the average daily feed intake of the elderly dogs (P < 0.01) and the average daily weight gain of all dogs (P < 0.05), enhanced the level of serum IgG (P < 0.001), IFN-α (P < 0.05), and fecal SIgA (P < 0.001), while reduced the TNF-α (P < 0.05). Additionally, probiotics could change the gut microbial structure of elderly dogs and significantly increased beneficial bacteria (including some Lactobacillus species and Faecalibacterium prausnitzii) and decreased potentially harmful bacteria (including Escherichia coli and Sutterella stercoricanisin), and the elderly dogs showed the strongest response to the probiotics; the relative abundance of some of these species correlated with certain immune factors and physiological parameters, suggesting that the probiotic treatment improved the host health and enhanced the host immunity by stimulating antibody and cytokine secretion through regulating canine gut microbiota. Furthermore, the gut microbiota of the elderly dogs shifted toward a younger-like composition at day 60 of probiotic treatment. Our findings suggested that the probiotic treatment effects on canine health and immunity were age-related and have provided interesting insights into future development of probiotic-based strategies to improve animal and human health.
Keywords: canine; health; immunity; intestinal microbiota; probiotics.
Figures

Similar articles
-
Metagenomic analysis revealed beneficial effects of probiotics in improving the composition and function of the gut microbiota in dogs with diarrhoea.Food Funct. 2019 May 22;10(5):2618-2629. doi: 10.1039/c9fo00087a. Food Funct. 2019. PMID: 31021333
-
Adjunctive treatment with probiotics partially alleviates symptoms and reduces inflammation in patients with irritable bowel syndrome.Eur J Nutr. 2021 Aug;60(5):2553-2565. doi: 10.1007/s00394-020-02437-4. Epub 2020 Nov 22. Eur J Nutr. 2021. PMID: 33225399 Clinical Trial.
-
Feed-additive probiotics accelerate yet antibiotics delay intestinal microbiota maturation in broiler chicken.Microbiome. 2017 Aug 3;5(1):91. doi: 10.1186/s40168-017-0315-1. Microbiome. 2017. PMID: 28768551 Free PMC article.
-
Weight gain by gut microbiota manipulation in productive animals.Microb Pathog. 2017 May;106:162-170. doi: 10.1016/j.micpath.2016.11.002. Epub 2016 Nov 9. Microb Pathog. 2017. PMID: 27836763 Review.
-
The canine gastrointestinal microbiota: early studies and research frontiers.Gut Microbes. 2020 Jul 3;11(4):635-654. doi: 10.1080/19490976.2019.1704142. Epub 2020 Jan 28. Gut Microbes. 2020. PMID: 31992112 Free PMC article. Review.
Cited by
-
Long-read metagenomics retrieves complete single-contig bacterial genomes from canine feces.BMC Genomics. 2021 May 6;22(1):330. doi: 10.1186/s12864-021-07607-0. BMC Genomics. 2021. PMID: 33957869 Free PMC article.
-
Gut Probiotics and Health of Dogs and Cats: Benefits, Applications, and Underlying Mechanisms.Microorganisms. 2023 Sep 29;11(10):2452. doi: 10.3390/microorganisms11102452. Microorganisms. 2023. PMID: 37894110 Free PMC article. Review.
-
Hypoglycemic Effects of Lycium barbarum Polysaccharide in Type 2 Diabetes Mellitus Mice via Modulating Gut Microbiota.Front Nutr. 2022 Jun 30;9:916271. doi: 10.3389/fnut.2022.916271. eCollection 2022. Front Nutr. 2022. PMID: 35845787 Free PMC article.
-
Lactiplantibacillus plantarum, lactiplantibacillus pentosus and inulin meal inclusion boost the metagenomic function of broiler chickens.Anim Microbiome. 2023 Aug 3;5(1):36. doi: 10.1186/s42523-023-00257-5. Anim Microbiome. 2023. PMID: 37537673 Free PMC article.
-
Probiotic-directed modulation of gut microbiota is basal microbiome dependent.Gut Microbes. 2020 Nov 9;12(1):1736974. doi: 10.1080/19490976.2020.1736974. Epub 2020 Mar 21. Gut Microbes. 2020. PMID: 32200683 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

