Complement Therapeutics in Autoimmune Disease
- PMID: 31001274
- PMCID: PMC6456694
- DOI: 10.3389/fimmu.2019.00672
Complement Therapeutics in Autoimmune Disease
Abstract
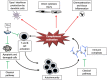
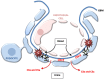
Many autoimmune diseases are characterized by generation of autoantibodies that bind to host proteins or deposit within tissues as a component of immune complexes. The autoantibodies can activate the complement system, which can mediate tissue damage and trigger systemic inflammation. Complement inhibitory drugs may, therefore, be beneficial across a large number of different autoimmune diseases. Many new anti-complement drugs that target specific activation mechanisms or downstream activation fragments are in development. Based on the shared pathophysiology of autoimmune diseases, some of these complement inhibitory drugs may provide benefit across multiple different diseases. In some antibody-mediated autoimmune diseases, however, unique features of the autoantibodies, the target antigens, or the affected tissues may make it advantageous to block individual components or pathways of the complement system. This paper reviews the evidence that complement is involved in various autoimmune diseases, as well as the studies that have examined whether or not complement inhibitors are effective for treating these diseases.
Keywords: antibody; autoimmunity; complement; immune complex; therapeutic.
Figures

Similar articles
-
Complement in therapy and disease: Regulating the complement system with antibody-based therapeutics.Mol Immunol. 2015 Oct;67(2 Pt A):117-30. doi: 10.1016/j.molimm.2015.01.028. Epub 2015 Feb 17. Mol Immunol. 2015. PMID: 25697848 Review.
-
Chemical Approaches to Modulating Complement-Mediated Diseases.J Med Chem. 2018 Apr 26;61(8):3253-3276. doi: 10.1021/acs.jmedchem.7b00882. Epub 2017 Oct 18. J Med Chem. 2018. PMID: 28977749 Review.
-
The role of complement in autoimmune renal disease.Autoimmunity. 2006 Aug;39(5):411-5. doi: 10.1080/08916930600739688. Autoimmunity. 2006. PMID: 16923541 Review.
-
The Evolving Landscape for Complement Therapeutics in Rheumatic and Autoimmune Diseases.Arthritis Rheumatol. 2017 Nov;69(11):2102-2113. doi: 10.1002/art.40219. Epub 2017 Oct 17. Arthritis Rheumatol. 2017. PMID: 28732131 Free PMC article. Review.
-
The immunoglobulin, IgG Fc receptor and complement triangle in autoimmune diseases.Immunobiology. 2012 Nov;217(11):1067-79. doi: 10.1016/j.imbio.2012.07.015. Immunobiology. 2012. PMID: 22964232 Review.
Cited by
-
Therapeutic targeting of the complement system.Nat Rev Drug Discov. 2019 Dec 9:10.1038/s41573-019-0055-y. doi: 10.1038/s41573-019-0055-y. Online ahead of print. Nat Rev Drug Discov. 2019. PMID: 31819218 Free PMC article.
-
Innate immunity and microbial dysbiosis in hidradenitis suppurativa - vicious cycle of chronic inflammation.Front Immunol. 2022 Jul 28;13:960488. doi: 10.3389/fimmu.2022.960488. eCollection 2022. Front Immunol. 2022. PMID: 35967376 Free PMC article. Review.
-
The Role of Adipsin, Complement Factor D, in the Pathogenesis of Graves' Orbitopathy.Invest Ophthalmol Vis Sci. 2023 Aug 1;64(11):13. doi: 10.1167/iovs.64.11.13. Invest Ophthalmol Vis Sci. 2023. PMID: 37555734 Free PMC article.
-
Efficacy and safety of gut microbiota-based therapies in autoimmune and rheumatic diseases: a systematic review and meta-analysis of 80 randomized controlled trials.BMC Med. 2024 Mar 13;22(1):110. doi: 10.1186/s12916-024-03303-4. BMC Med. 2024. PMID: 38475833 Free PMC article.
-
Advantages of using complement components in preventive and therapeutic vaccine strategies for infectious and non-infectious diseases.J R Soc Interface. 2025 Jul;22(228):20250138. doi: 10.1098/rsif.2025.0138. Epub 2025 Jul 2. J R Soc Interface. 2025. PMID: 40592462 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

