Induction of Fc-Mediated Effector Functions Against a Stabilized Inner Domain of HIV-1 gp120 Designed to Selectively Harbor the A32 Epitope Region
- PMID: 31001276
- PMCID: PMC6455405
- DOI: 10.3389/fimmu.2019.00677
Induction of Fc-Mediated Effector Functions Against a Stabilized Inner Domain of HIV-1 gp120 Designed to Selectively Harbor the A32 Epitope Region
Abstract
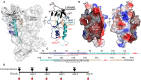
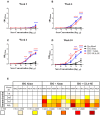
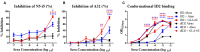
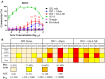
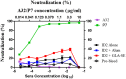
Recent clinical trials and studies using nonhuman primates (NHPs) suggest that antibody-mediated protection against HIV-1 will require α-HIV envelope humoral immunity beyond direct neutralization to include Fc-receptor (FcR) mediated effector functions such as antibody-dependent cellular cytotoxicity (ADCC). There is also strong evidence indicating that the most potent ADCC response in humans is directed toward transitional non-neutralizing epitopes associated with the gp41-interactive face of gp120, particularly those within the first and second constant (C1-C2) region (A32-like epitopes). These epitopes were shown to be major targets of ADCC responses during natural infection and have been implicated in vaccine-induced protective immunity. Here we describe the immunogenicity of ID2, an immunogen consisting of the inner domain of the clade A/E 93TH057 HIV-1 gp120 expressed independently of the outer domain (OD) and stabilized in the CD4-bound conformation to harbor conformational A32 region epitopes within a minimal structural unit of HIV-1 Env. ID2 induced A32-specific antibody responses in BALB/c mice when injected alone or in the presence of the adjuvants Alum or GLA-SE. Low α-ID2 titers were detected in mice immunized with ID2 alone whereas robust responses were observed with ID2 plus adjuvant, with the greatest ID2 and A32-specific titers observed in the GLA-SE group. Only sera from groups immunized in the presence of GLA-SE were capable of mediating significant ADCC using NKr cells sensitized with recombinant BaL gp120 as targets and human PBMCs as effectors. A neutralization response to a tier 2 virus was not observed. Altogether, our studies demonstrate that ID2 is highly immunogenic and elicits A32-specific ADCC responses in an animal host. The ID2 immunogen has significant translational value as it can be used in challenge studies to evaluate the role of non-neutralizing antibodies directed at the A32 subregion in HIV-1 protection.
Keywords: A32 epitope; ADCC (Antibody dependent cellular cytotoxicity); Fc-mediated effector function; HIV envelope; ID (Inner domain) immunogen.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

