Traumatic Injury and Exposure to Mitochondrial-Derived Damage Associated Molecular Patterns Suppresses Neutrophil Extracellular Trap Formation
- PMID: 31001279
- PMCID: PMC6455291
- DOI: 10.3389/fimmu.2019.00685
Traumatic Injury and Exposure to Mitochondrial-Derived Damage Associated Molecular Patterns Suppresses Neutrophil Extracellular Trap Formation
Abstract
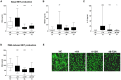
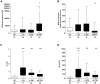
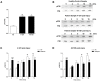
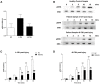
Major traumatic injury induces significant remodeling of the circulating neutrophil pool and loss of bactericidal function. Although a well-described phenomenon, research to date has only analyzed blood samples acquired post-hospital admission, and the mechanisms that initiate compromised neutrophil function post-injury are therefore poorly understood. Here, we analyzed pre-hospital blood samples acquired from 62 adult trauma patients (mean age 44 years, range 19-95 years) within 1 h of injury (mean time to sample 39 min, range 13-59 min). We found an immediate impairment in neutrophil extracellular trap (NET) generation in response to phorbol 12-myristate 13-acetate (PMA) stimulation, which persisted into the acute post-injury phase (4-72 h). Reduced NET generation was accompanied by reduced reactive oxygen species production, impaired activation of mitogen-activated protein kinases, and a reduction in neutrophil glucose uptake and metabolism to lactate. Pre-treating neutrophils from healthy subjects with mitochondrial-derived damage-associated molecular patterns (mtDAMPs), whose circulating levels were significantly increased in our trauma patients, reduced NET generation. This mtDAMP-induced impairment in NET formation was associated with an N-formyl peptide mediated activation of AMP-activated protein kinase (AMPK), a negative regulator of aerobic glycolysis and NET formation. Indeed, activation of AMPK via treatment with the AMP-mimetic AICAR significantly reduced neutrophil lactate production in response to PMA stimulation, a phenomenon that we also observed for neutrophils pre-treated with mtDAMPs. Furthermore, the impairment in NET generation induced by mtDAMPs was partially ameliorated by pre-treating neutrophils with the AMPK inhibitor compound C. Taken together, our data demonstrate an immediate trauma-induced impairment in neutrophil anti-microbial function and identify mtDAMP release as a potential initiator of acute post-injury neutrophil dysfunction.
Keywords: immune suppression; mitochondrial-derived DAMPs; neutrophil extracellular traps; neutrophils; trauma.
Figures



Similar articles
-
N-Formyl peptides drive mitochondrial damage associated molecular pattern induced neutrophil activation through ERK1/2 and P38 MAP kinase signalling pathways.Injury. 2015;46(6):975-84. doi: 10.1016/j.injury.2015.03.028. Epub 2015 Mar 17. Injury. 2015. PMID: 25817163
-
The impact of trauma relevant concentrations of prostaglandin E2 on the anti-microbial activity of the innate immune system.Front Immunol. 2024 Oct 22;15:1401185. doi: 10.3389/fimmu.2024.1401185. eCollection 2024. Front Immunol. 2024. PMID: 39502706 Free PMC article.
-
Mitochondrial DNA released by trauma induces neutrophil extracellular traps.PLoS One. 2015 Mar 16;10(3):e0120549. doi: 10.1371/journal.pone.0120549. eCollection 2015. PLoS One. 2015. PMID: 25774524 Free PMC article.
-
Mitochondrial DNA neutrophil extracellular traps are formed after trauma and subsequent surgery.J Crit Care. 2014 Dec;29(6):1133.e1-5. doi: 10.1016/j.jcrc.2014.07.013. Epub 2014 Jul 22. J Crit Care. 2014. PMID: 25128442 Review.
-
Neutrophil extracellular traps as a new paradigm in innate immunity: friend or foe?Periodontol 2000. 2013 Oct;63(1):165-97. doi: 10.1111/prd.12025. Periodontol 2000. 2013. PMID: 23931060 Review.
Cited by
-
The role of neutrophil extracellular traps in acute lung injury.Front Immunol. 2022 Jul 29;13:953195. doi: 10.3389/fimmu.2022.953195. eCollection 2022. Front Immunol. 2022. PMID: 35967320 Free PMC article. Review.
-
Neutrophils and COVID-19: Active Participants and Rational Therapeutic Targets.Front Immunol. 2021 Jun 2;12:680134. doi: 10.3389/fimmu.2021.680134. eCollection 2021. Front Immunol. 2021. PMID: 34149717 Free PMC article. Review.
-
The Metabolic Basis of Immune Dysfunction Following Sepsis and Trauma.Front Immunol. 2020 May 29;11:1043. doi: 10.3389/fimmu.2020.01043. eCollection 2020. Front Immunol. 2020. PMID: 32547553 Free PMC article. Review.
-
Early increase in anti-inflammatory biomarkers is associated with the development of multiple organ dysfunction syndrome in severely injured trauma patients.Trauma Surg Acute Care Open. 2019 Oct 23;4(1):e000343. doi: 10.1136/tsaco-2019-000343. eCollection 2019. Trauma Surg Acute Care Open. 2019. PMID: 31750398 Free PMC article.
-
Major Traumatic Injury and Exposure to Mitochondrial-Derived Damage-Associated Molecular Patterns Promotes Neutrophil Survival Accompanied by Stabilisation of the Anti-Apoptotic Protein Mcl-1.Cells. 2025 May 21;14(10):754. doi: 10.3390/cells14100754. Cells. 2025. PMID: 40422258 Free PMC article.
References
-
- Hampson P, Dinsdale RJ, Wearn CM, Bamford AL, Bishop JRB, Hazeldine J, et al. . Neutrophil dysfunction, immature granulocytes, and cell-free DNA are early biomarkers of sepsis in burn-injured patients: a prospective observational cohort study. Ann Surg. (2017) 265:1241–9. 10.1097/SLA.0000000000001807 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

