The Rise of Apomixis in Natural Plant Populations
- PMID: 31001296
- PMCID: PMC6454013
- DOI: 10.3389/fpls.2019.00358
The Rise of Apomixis in Natural Plant Populations
Abstract
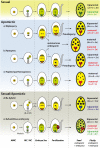
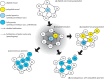
Apomixis, the asexual reproduction via seed, has many potential applications for plant breeding by maintaining desirable genotypes over generations. Since most major crops do not express natural apomixis, it is useful to understand the origin and maintenance of apomixis in natural plant systems. Here, we review the state of knowledge on origin, establishment and maintenance of natural apomixis. Many studies suggest that hybridization, either on diploid or polyploid cytotypes, is a major trigger for the formation of unreduced female gametophytes, which represents the first step toward apomixis, and must be combined to parthenogenesis, the development of an unfertilized egg cell. Nevertheless, fertilization of endosperm is still needed for most apomictic plants. Coupling of these three steps appears to be a major constraint for shifts to natural apomixis. Adventitious embryony is another developmental pathway toward apomixis. Establishment of a newly arisen apomictic lineage is often fostered by side-effects of polyploidy. Polyploidy creates an immediate reproductive barrier against the diploid parental and progenitor populations; it can cause a breakdown of genetic self-incompatibility (SI) systems which is needed to establish self-fertility of pseudogamous apomictic lineages; and finally, polyploidy could indirectly help to establish an apomictic cytotype in a novel ecological niche by increasing adaptive potentials of the plants. This step may be followed by a phase of diversification and range expansion, mostly described as geographical parthenogenesis. The utilization of apomixis in crops must consider the potential risks of pollen transfer and introgression into sexual crop fields, which might be overcome by using pollen-sterile or cleistogamous variants. Another risk is the escape into natural vegetation and potential invasiveness of apomictic plants which needs careful management and consideration of ecological conditions.
Keywords: apomictic crops; grass cultivars; polyploidy; reproductive assurance; sexuality; speciation; triploid bridge.
Figures
Similar articles
-
Effects of apomixis and polyploidy on diversification and geographic distribution in Amelanchier (Rosaceae).Am J Bot. 2014 Aug;101(8):1375-87. doi: 10.3732/ajb.1400113. Am J Bot. 2014. PMID: 25156985
-
Introgression of apomixis into sexual species is inhibited by mentor effects and ploidy barriers in the Ranunculus auricomus complex.Ann Bot. 2009 Jul;104(1):81-9. doi: 10.1093/aob/mcp093. Epub 2009 Apr 22. Ann Bot. 2009. PMID: 19386790 Free PMC article.
-
Reproductive differentiation into sexual and apomictic polyploid cytotypes in Potentilla puberula (Potentilleae, Rosaceae).Ann Bot. 2013 Oct;112(6):1159-68. doi: 10.1093/aob/mct167. Epub 2013 Aug 19. Ann Bot. 2013. PMID: 23960045 Free PMC article.
-
The evolution of self-fertility in apomictic plants.Sex Plant Reprod. 2010 Mar;23(1):73-86. doi: 10.1007/s00497-009-0122-3. Epub 2009 Nov 20. Sex Plant Reprod. 2010. PMID: 20165965 Free PMC article. Review.
-
The genetic control of apomixis: asexual seed formation.Genetics. 2014 Jun;197(2):441-50. doi: 10.1534/genetics.114.163105. Genetics. 2014. PMID: 24939990 Free PMC article. Review.
Cited by
-
Reproduction Modes and Conservation Implications in Three Polyploid Sorbus Stenoendemics in Eastern Slovakia (Central Europe).Plants (Basel). 2023 Jan 13;12(2):373. doi: 10.3390/plants12020373. Plants (Basel). 2023. PMID: 36679086 Free PMC article.
-
Environmental and Genetic Factors Affecting Apospory Expressivity in Diploid Paspalum rufum.Plants (Basel). 2021 Oct 4;10(10):2100. doi: 10.3390/plants10102100. Plants (Basel). 2021. PMID: 34685909 Free PMC article.
-
The Application of Flow Cytometry for Estimating Genome Size, Ploidy Level Endopolyploidy, and Reproductive Modes in Plants.Methods Mol Biol. 2021;2222:325-361. doi: 10.1007/978-1-0716-0997-2_17. Methods Mol Biol. 2021. PMID: 33301101
-
Structural variation and parallel evolution of apomixis in citrus during domestication and diversification.Natl Sci Rev. 2022 Jun 14;9(10):nwac114. doi: 10.1093/nsr/nwac114. eCollection 2022 Oct. Natl Sci Rev. 2022. PMID: 36415319 Free PMC article.
-
Genomic insights into the clonal reproductive Opuntia cochenillifera: mitochondrial and chloroplast genomes of the cochineal cactus for enhanced understanding of structural dynamics and evolutionary implications.Front Plant Sci. 2024 Mar 7;15:1347945. doi: 10.3389/fpls.2024.1347945. eCollection 2024. Front Plant Sci. 2024. PMID: 38516667 Free PMC article.
References
-
- Almeida-Neto M., Prado P. I., Kubota U., Bariani J. M., Aguirre G. A., Lewinsohn T. M. (2010). Invasive grasses and native Asteraceae in the Brazilian Cerrado. Plant Ecol. 209 109–122. 10.1007/s11258-010-9727-8 - DOI
-
- Alves M. F., Duarte M. O., Bittencourt N. S., Oliveira P. E., Sampaio D. S. (2016). Sporophytic apomixis in polyembryonic Handroanthus serratifolius (Vahl) SO Grose (Bignoniaceae) characterizes the species as an agamic polyploid complex. Plant Syst. Evol. 302 651–659. 10.1007/s00606-016-1291-1299 - DOI
-
- Arnaud J. F., Viard F., Delescluse M., Cuguen J. (2003). Evidence for gene flow via seed dispersal from crop to wild relatives in Beta vulgaris (Chenopodiaceae): consequences for the release of genetically modified crop species with weedy lineages. Proc. Biol. Sci. 270 1565–1571. 10.1098/rspb.2003.2407 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

