Laboratory Evolution of a Saccharomyces cerevisiae × S. eubayanus Hybrid Under Simulated Lager-Brewing Conditions
- PMID: 31001314
- PMCID: PMC6455053
- DOI: 10.3389/fgene.2019.00242
Laboratory Evolution of a Saccharomyces cerevisiae × S. eubayanus Hybrid Under Simulated Lager-Brewing Conditions
Abstract
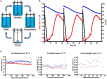
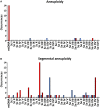
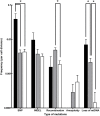
Saccharomyces pastorianus lager-brewing yeasts are domesticated hybrids of S. cerevisiae x S. eubayanus that display extensive inter-strain chromosome copy number variation and chromosomal recombinations. It is unclear to what extent such genome rearrangements are intrinsic to the domestication of hybrid brewing yeasts and whether they contribute to their industrial performance. Here, an allodiploid laboratory hybrid of S. cerevisiae and S. eubayanus was evolved for up to 418 generations on wort under simulated lager-brewing conditions in six independent sequential batch bioreactors. Characterization of 55 single-cell isolates from the evolved cultures showed large phenotypic diversity and whole-genome sequencing revealed a large array of mutations. Frequent loss of heterozygosity involved diverse, strain-specific chromosomal translocations, which differed from those observed in domesticated, aneuploid S. pastorianus brewing strains. In contrast to the extensive aneuploidy of domesticated S. pastorianus strains, the evolved isolates only showed limited (segmental) aneuploidy. Specific mutations could be linked to calcium-dependent flocculation, loss of maltotriose utilization and loss of mitochondrial activity, three industrially relevant traits that also occur in domesticated S. pastorianus strains. This study indicates that fast acquisition of extensive aneuploidy is not required for genetic adaptation of S. cerevisiae × S. eubayanus hybrids to brewing environments. In addition, this work demonstrates that, consistent with the diversity of brewing strains for maltotriose utilization, domestication under brewing conditions can result in loss of this industrially relevant trait. These observations have important implications for the design of strategies to improve industrial performance of novel laboratory-made hybrids.
Keywords: Saccharomyces pastorianus; domestication; flocculation; laboratory evolution; loss of heterozygosity; maltotriose utilization.
Figures

Similar articles
-
Himalayan Saccharomyces eubayanus Genome Sequences Reveal Genetic Markers Explaining Heterotic Maltotriose Consumption by Saccharomyces pastorianus Hybrids.Appl Environ Microbiol. 2019 Oct 30;85(22):e01516-19. doi: 10.1128/AEM.01516-19. Print 2019 Nov 15. Appl Environ Microbiol. 2019. PMID: 31519660 Free PMC article.
-
Industrially Applicable De Novo Lager Yeast Hybrids with a Unique Genomic Architecture: Creation and Characterization.Appl Environ Microbiol. 2021 Jan 15;87(3):e02434-20. doi: 10.1128/AEM.02434-20. Print 2021 Jan 15. Appl Environ Microbiol. 2021. PMID: 33188002 Free PMC article.
-
The Genome Sequence of Saccharomyces eubayanus and the Domestication of Lager-Brewing Yeasts.Mol Biol Evol. 2015 Nov;32(11):2818-31. doi: 10.1093/molbev/msv168. Epub 2015 Aug 11. Mol Biol Evol. 2015. PMID: 26269586 Free PMC article.
-
Lager-brewing yeasts in the era of modern genetics.FEMS Yeast Res. 2019 Nov 1;19(7):foz063. doi: 10.1093/femsyr/foz063. FEMS Yeast Res. 2019. PMID: 31553794 Free PMC article. Review.
-
Novel brewing yeast hybrids: creation and application.Appl Microbiol Biotechnol. 2017 Jan;101(1):65-78. doi: 10.1007/s00253-016-8007-5. Epub 2016 Nov 24. Appl Microbiol Biotechnol. 2017. PMID: 27885413 Free PMC article. Review.
Cited by
-
Ploidy evolution in a wild yeast is linked to an interaction between cell type and metabolism.PLoS Biol. 2023 Nov 9;21(11):e3001909. doi: 10.1371/journal.pbio.3001909. eCollection 2023 Nov. PLoS Biol. 2023. PMID: 37943740 Free PMC article.
-
Positive selection of efficient ethanol producers from xylose at 45 °C in the yeast Ogataea polymorpha.Sci Rep. 2025 Jul 22;15(1):26530. doi: 10.1038/s41598-025-12204-2. Sci Rep. 2025. PMID: 40691245 Free PMC article.
-
Fermentation innovation through complex hybridization of wild and domesticated yeasts.Nat Ecol Evol. 2019 Nov;3(11):1576-1586. doi: 10.1038/s41559-019-0998-8. Epub 2019 Oct 21. Nat Ecol Evol. 2019. PMID: 31636426 Free PMC article.
-
Phenotype-Independent Isolation of Interspecies Saccharomyces Hybrids by Dual-Dye Fluorescent Staining and Fluorescence-Activated Cell Sorting.Front Microbiol. 2019 Apr 26;10:871. doi: 10.3389/fmicb.2019.00871. eCollection 2019. Front Microbiol. 2019. PMID: 31105669 Free PMC article.
-
Does Inter-Organellar Proteostasis Impact Yeast Quality and Performance During Beer Fermentation?Front Genet. 2020 Jan 31;11:2. doi: 10.3389/fgene.2020.00002. eCollection 2020. Front Genet. 2020. PMID: 32076433 Free PMC article.
References
-
- Alves S. L., Herberts R. A., Hollatz C., Trichez D., Miletti L. C., De Araujo P. S., et al. . (2008). Molecular analysis of maltotriose active transport and fermentation by Saccharomyces cerevisiae reveals a determinant role for the AGT1 permease. Appl. Environ. Microbiol. 74, 1494–1501. 10.1128/AEM.02570-07 - DOI - PMC - PubMed
-
- Andreasen A. A., Stier T. (1953). Anaerobic nutrition of Saccharomyces cerevisiae. I. ergosterol requirement for growth in a defined medium. J. Cell. Comp. Physiol. 41, 23–36. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

