Human Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles Inhibit Endometrial Cancer Cell Proliferation and Migration through Delivery of Exogenous miR-302a
- PMID: 31001342
- PMCID: PMC6437733
- DOI: 10.1155/2019/8108576
Human Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles Inhibit Endometrial Cancer Cell Proliferation and Migration through Delivery of Exogenous miR-302a
Abstract
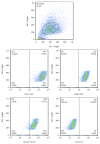
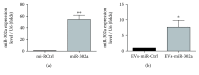
MicroRNAs (miRNAs) are potential therapeutic targets in endometrial cancer, but the difficulties associated with their delivery to tumor target cells have hampered their applications. Human umbilical cord mesenchymal stem cells (hUCMSCs) have a well-recognized tumor-homing ability, emphasizing the capacity of tumor-targeted delivery of extracellular vesicles. hUCMSCs release extracellular vesicles rich in miRNAs, which play a vital role in intercellular communication. The purpose of this study was to verify a potential tumor suppressor microRNA, miR-302a, and engineered hUCMSC extracellular vesicles enriched with miR-302a for therapy of endometrial cancer. Here, we observed that miR-302a was significantly downregulated in endometrial cancer tissues when compared with adjacent tissues. Overexpression of miR-302a in endometrial cancer cells robustly suppressed cell proliferation and migration. Meanwhile, the proliferation and migration were significantly inhibited in endometrial cancer cells when cultured with miR-302a-loaded extracellular vesicles derived from hUCMSCs. Importantly, our data showed that engineered extracellular vesicles rich in miR-302 significantly inhibited the expression of cyclin D1 and suppressed AKT signaling pathway in endometrial cancer cells. These results suggested that exogenous miR-302a delivered by hUCMSC-derived extracellular vesicles has exciting potential as an effective anticancer therapy.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

