Alpinia oxyphylla Fruit Extract Ameliorates Experimental Autoimmune Encephalomyelitis through the Regulation of Th1/Th17 Cells
- PMID: 31001353
- PMCID: PMC6437745
- DOI: 10.1155/2019/6797030
Alpinia oxyphylla Fruit Extract Ameliorates Experimental Autoimmune Encephalomyelitis through the Regulation of Th1/Th17 Cells
Abstract
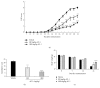
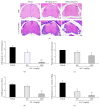
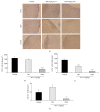
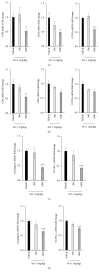
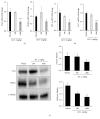
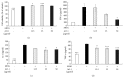
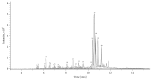
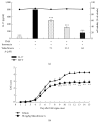
Alpinia oxyphylla is a traditional Chinese medicine widely used for treating diarrhea, ulceration, and enuresis. Moreover, A. oxyphylla is effective for cognitive function improvement and nerve regeneration. Multiple sclerosis (MS) is a chronic neuronal inflammatory autoimmune disease that commonly affects young adults in high-latitude regions. The aim of this study was to evaluate the beneficial effects of A. oxyphylla in an experimental autoimmune encephalomyelitis (EAE) mouse model, which is an extensively used model for human MS. The ethanolic extract of A. oxyphylla fruit (AO-1) was orally administered to EAE mice. Our results showed AO-1 significantly reduced EAE symptoms. Histopathological analysis showed AO-1 reduced demyelination, inflammation, gliosis, and axonal swelling in the spinal cord. Furthermore, immunohistochemistry and quantitative polymerase chain reaction (qPCR) studies revealed that the infiltration of CD4+, CD8+ T cells, and CD11b+ monocytes into the spinal cord decreased in the AO-1-treated group. Mechanistically, the Th1 transcription factor T-bet, Th17 transcription factor retinoic acid receptor-related orphan receptor γ (RORγt), and inflammatory cytokines interferon (IFN)-γ and interleukin (IL)-17 were reduced in the spinal cords of mice treated with AO-1. The expression levels of T-bet and RORγt were also lowered in the spleens of those mice. Further in vitro study showed AO-1 inhibited production of IFN-γ, IL-2, and tumor necrosis factor-α from MOG35-55-peptide-stimulated splenocytes. One component isolated from AO-1, yakuchinone A, inhibited IL-17 production in vitro and reduced EAE symptoms in the mice. Collectively, our results indicate that AO-1 ameliorated the severity of EAE in mice and may involve the regulation of Th1/Th17 response. A. oxyphylla warrants further investigation, particularly regarding its clinical benefits for MS.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials

