Strongly correlated oxides for energy harvesting
- PMID: 31001365
- PMCID: PMC6454405
- DOI: 10.1080/14686996.2018.1529524
Strongly correlated oxides for energy harvesting
Abstract
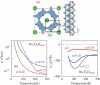
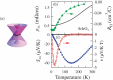
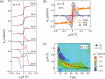
We review recent advances in strongly correlated oxides as thermoelectric materials in pursuit of energy harvesting. We discuss two topics: one is the enhancement of the ordinary thermoelectric properties by controlling orbital degrees of freedom and orbital fluctuation not only in bulk but also at the interface of correlated oxides. The other topic is the use of new phenomena driven by spin-orbit coupling (SOC) of materials. In 5d electron oxides, we show some SOC-related transport phenomena, which potentially contribute to energy harvesting. We outline the current status and a future perspective of oxides as thermoelectric materials.
Keywords: 210 Thermoelectronics / Thermal transport / insulators; 50 Energy Materials; Dirac semimetal; Seebeck effect; Thermoelectric materials; Weyl semimetal; anomalous Nernst effect; magnetic skyrmion; spin Hall effect; spin Seebeck effect; strongly correlated oxides.
Figures





References
-
- Terasaki I, Sasago Y, Uchinokura K.. Large thermoelectric power in NaCo2O4 single crystals. Phys Rev B. 1997;56:R12685–R12687.
-
- Koshibae W, Tsutsui K, Maekawa S.. Thermopower in cobalt oxides. Phys Rev B. 2000;62:6869–6872.
-
- Kuroki K, Arita R. “Pudding mold” band drives large thermopower in NaxCoO2. J Phys Soc Jpn. 2007;76:083707.
-
- Yang HB, Wang SC, Sekharan AKP, et al. ARPES on Na0.6CoO2: fermi surface and unusual band dispersion. Phys Rev Lett. 2004;92:246403. - PubMed
-
- Funahashi R, Matsubara I, Ikuta H, et al. An oxide single crystal with high thermoelectric performance in air. Jpn J Appl Phys. 2000;39:L1127–L1129.
Publication types
LinkOut - more resources
Full Text Sources
