Clinical Experience with Hemopatch® as a Dural Sealant in Cranial Neurosurgery
- PMID: 31001467
- PMCID: PMC6450590
- DOI: 10.7759/cureus.4013
Clinical Experience with Hemopatch® as a Dural Sealant in Cranial Neurosurgery
Abstract
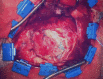
Background: Herein, we report our clinical experience with the novel polyethylene glycol-covered matrix dural onlay, Hemopatch® (Baxter Deutschland GmbH, Unterschleißheim, Germany) for the prevention of postoperative cerebrospinal fluid (CSF) fistulas.
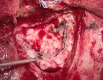
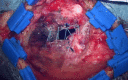
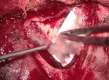
Methods: Retrospectively, 22 consecutive patients (11 females, 11 males, mean age: 49.8 years, range: 15-77 years) with oncological and vascular intracranial lesions were included in this study. In all patients, the Hemopatch was applied as the dural onlay. The accuracy of the primary dural sutures was distinguished into 1) no visible gaps, 2) small gaps < 3 mm, and 3) large gaps > 3 mm. We evaluated the patient charts, surgical reports, and postoperative images. The median follow-up was three months. We recorded any wound healing disorder, such as infection or CSF fistula, and postoperative hemorrhage resulting in surgical revision.
Results: Supratentorial, infratentorial, and transsphenoidal approaches were conducted in 17, four, and one patient, respectively. Accurate sutures without visible gaps, small gaps, and large gaps were covered with the Hemopatch in 11, eight, and three patients. One patient developed a CSF fistula (4.5%), one patient had a wound infection (4.5%), and in one patient, a remote cerebellar hemorrhage occurred (unrelated to the dural closure) (4.5%). Thus, the surgical revision rate due to wound healing disorders was 9% (2/22).
Conclusion: It is safe and feasible to use the Hemopatch as a dural sealant. The rate of postoperative wound healing disorders in our population was in the lower range of reported surgical revision rates after supra-/infratentorial craniotomies. However, prospective and controlled clinical trials are still warranted.
Keywords: dura substitute; dural sealant; hemopatch.
Conflict of interest statement
The authors have declared financial relationships, which are detailed in the next section.
Figures
References
-
- Risk factors for postoperative CSF leakage after elective craniotomy and the efficacy of fleece-bound tissue sealing against dural suturing alone: a randomized controlled trial. Hutter G, von Felten S, Sailer MH, Schulz M, Mariani L. J Neurosurg. 2014;121:735–744. - PubMed
-
- Evaluation of the use of BioGlue in neurosurgical procedures. Kumar A, Maartens NF, Kaye AH. J Clin Neurosci. 2003;10:661–664. - PubMed
-
- Prevention and management of cerebrospinal fluid leak following vestibular schwannoma surgery. Fishman AJ, Marrinan MS, Golfinos JG, Cohen NL, Roland JT Jr. Laryngoscope. 2004;114:501–505. - PubMed
-
- Management of 1000 vestibular schwannomas (acoustic neuromas): the facial nerve--preservation and restitution of function. Samii M, Matthies C. Neurosurgery. 1997;40:684–694. - PubMed
-
- Risk factors of neurosurgical site infection after craniotomy: a systematic review and meta-analysis. Fang C, Zhu T, Zhang P, Xia L, Sun C. Am J Infect Control. 2017;45:123–134. - PubMed
LinkOut - more resources
Full Text Sources