Fluoro-Aryl Substituted α,β2,3-Peptides in the Development of Foldameric Antiparallel β-Sheets: A Conformational Study
- PMID: 31001518
- PMCID: PMC6454073
- DOI: 10.3389/fchem.2019.00192
Fluoro-Aryl Substituted α,β2,3-Peptides in the Development of Foldameric Antiparallel β-Sheets: A Conformational Study
Abstract
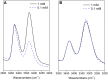
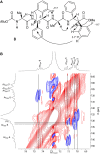
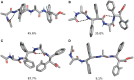
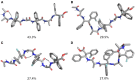
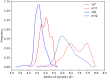
α,β2,3-Disteroisomeric foldamers of general formula Boc(S-Ala-β-2R,3R-Fpg)nOMe or Boc(S-Ala-β-2S,3S-Fpg)nOMe were prepared from both enantiomers of syn H-2-(2-F-Phe)-h-PheGly-OH (named β-Fpg) and S-alanine. Our peptides show two appealing features for biomedical applications: the presence of fluorine, attractive for non-covalent interactions, and aryl groups, crucial for π-stacking. A conformational study was performed, using IR, NMR and computational studies of diastereoisomeric tetra- and hexapeptides containing the β2,3-amino acid in the R,R- and S,S-stereochemistry, respectively. We found that the stability of peptide conformation is dependent on the stereochemistry of the β-amino acid. Combining S-Ala with β-2R,3R-Fpg, a stable extended β-strand conformation was obtained. Furthermore, β-2R,3R-Fpg containing hexapeptide self-assembles to form antiparallel β-sheet structure stabilized by intermolecular H-bonds and π,π-interactions. These features make peptides containing the β2,3-fluoro amino acid very appealing for the development of bioactive proteolytically stable foldameric β-sheets as modulators of protein-protein interaction (PPI).
Keywords: antiparallel β-sheet; conformational analyses; extended peptide; foldamers; α, β2,3-peptide; β2,3-diaryl-amino acid.
Figures
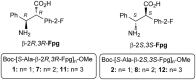




References
-
- Angelici G., Luppi G., Kaptein B., Broxterman Q. B., Hofmann H. J., Tomasini C. (2007). Synthesis and secondary structure of alternate α,β-hybrid peptides containing oxazolidin-2-one moieties. Eur. J. Org. Chem. 2007, 2713–2721. 10.1002/ejoc.200700134 - DOI
-
- Baldauf C., Hofmann H. J. (2012). Ab initio MO theory—An important tool in foldamer research: prediction of helices in oligomers of ω-amino acids. Helv. Chim. Acta 95, 2348–2383. 10.1002/hlca.201200436 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

