Fibronectin Functionalized Electrospun Fibers by Using Benign Solvents: Best Way to Achieve Effective Functionalization
- PMID: 31001528
- PMCID: PMC6456675
- DOI: 10.3389/fbioe.2019.00068
Fibronectin Functionalized Electrospun Fibers by Using Benign Solvents: Best Way to Achieve Effective Functionalization
Abstract
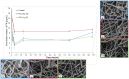
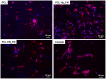
The aim of this study is to demonstrate the feasibility of different functionalization methods for electrospun fibers developed using benign solvents. In particular three different approaches were investigated to achieve the functionalization of poly(epsilon caprolactone) (PCL) electrospun fibers with fibronectin. Protein surface entrapment, chemical functionalization and coaxial electrospinning were performed and compared. Moreover, bilayered scaffolds, with a top patterned and functionalized layer with fibronectin and a randomly oriented not functionalized layer were fabricated, demonstrating the versatility of the use of benign solvents for electrospinning also for the fabrication of complex graded structures. Besides the characterization of the morphology of the obtained scaffolds, ATR-FTIR and ToF-SIMS were used for the surface characterization of the functionalized fibers. Cell adhesion and proliferation were also investigated by using ST-2 cells. Positive results were obtained from all functionalized scaffolds and the most promising results were obtained with bilayered scaffolds, in terms of cells infiltration inside the fibrous structure.
Keywords: benign solvents; electrospinning; fibronectin; functionalization; nanofibers; scaffolds; tissue engineering.
Figures










Similar articles
-
Incorporation of Calcium Containing Mesoporous (MCM-41-Type) Particles in Electrospun PCL Fibers by Using Benign Solvents.Polymers (Basel). 2017 Oct 4;9(10):487. doi: 10.3390/polym9100487. Polymers (Basel). 2017. PMID: 30965790 Free PMC article.
-
Thiol-ene conjugation of VEGF peptide to electrospun scaffolds as potential application for angiogenesis.Bioact Mater. 2022 Jun 8;20:306-317. doi: 10.1016/j.bioactmat.2022.05.029. eCollection 2023 Feb. Bioact Mater. 2022. PMID: 35755423 Free PMC article.
-
Versatile Production of Poly(Epsilon-Caprolactone) Fibers by Electrospinning Using Benign Solvents.Nanomaterials (Basel). 2016 Apr 15;6(4):75. doi: 10.3390/nano6040075. Nanomaterials (Basel). 2016. PMID: 28335202 Free PMC article.
-
Hydrophilic Surface Functionalization of Electrospun Nanofibrous Scaffolds in Tissue Engineering.Polymers (Basel). 2020 Nov 10;12(11):2636. doi: 10.3390/polym12112636. Polymers (Basel). 2020. PMID: 33182617 Free PMC article. Review.
-
The Use of Electrospinning Technique on Osteochondral Tissue Engineering.Adv Exp Med Biol. 2018;1058:247-263. doi: 10.1007/978-3-319-76711-6_11. Adv Exp Med Biol. 2018. PMID: 29691825 Review.
Cited by
-
Nanocomposite electrospun fibers of poly(ε-caprolactone)/bioactive glass with shape memory properties.Bioact Mater. 2021 Sep 23;11:230-239. doi: 10.1016/j.bioactmat.2021.09.020. eCollection 2022 May. Bioact Mater. 2021. PMID: 34977428 Free PMC article.
-
Electrospun PCL/PGS Composite Fibers Incorporating Bioactive Glass Particles for Soft Tissue Engineering Applications.Nanomaterials (Basel). 2020 May 19;10(5):978. doi: 10.3390/nano10050978. Nanomaterials (Basel). 2020. PMID: 32438673 Free PMC article.
-
Fibronectin Adsorption on Electrospun Synthetic Vascular Grafts Attracts Endothelial Progenitor Cells and Promotes Endothelialization in Dynamic In Vitro Culture.Cells. 2020 Mar 23;9(3):778. doi: 10.3390/cells9030778. Cells. 2020. PMID: 32210018 Free PMC article.
-
Electrospun Nanomaterials Based on Cellulose and Its Derivatives for Cell Cultures: Recent Developments and Challenges.Polymers (Basel). 2023 Feb 26;15(5):1174. doi: 10.3390/polym15051174. Polymers (Basel). 2023. PMID: 36904415 Free PMC article. Review.
-
Current Concepts and Methods in Tissue Interface Scaffold Fabrication.Biomimetics (Basel). 2022 Oct 4;7(4):151. doi: 10.3390/biomimetics7040151. Biomimetics (Basel). 2022. PMID: 36278708 Free PMC article. Review.
References
-
- Chakrapani V. Y., Gnanamani A., Giridev V. R., Madhusoothanan M., Sekaran G. (2012). Electrospinning of type I collagen and PCL nanofibers using acetic acid. J. Appl. Polymer Sci. 125, 3221–3227. 10.1002/app.36504 - DOI
-
- Chutipakdeevong J., Ruktanonchai U., Supaphol P. (2014). Hybrid biomimetic electrospun fibrous mats derived from poly(ε-caprolactone) and silk fibroin protein for wound dressing application. J. Appl. Polymer Sci. 132:41653 10.1002/app.41653 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

