Tubing-Free Microfluidic Microtissue Culture System Featuring Gradual, in vivo-Like Substance Exposure Profiles
- PMID: 31001529
- PMCID: PMC6454105
- DOI: 10.3389/fbioe.2019.00072
Tubing-Free Microfluidic Microtissue Culture System Featuring Gradual, in vivo-Like Substance Exposure Profiles
Abstract
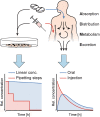
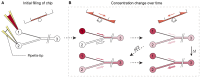
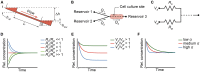
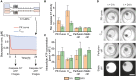
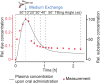
In vitro screening methods for compound efficacy and toxicity to date mostly include cell or tissue exposure to preset constant compound concentrations over a defined testing period. Such concentration profiles, however, do not represent realistic in vivo situations after substance uptake. Absorption, distribution, metabolism and excretion of administered substances in an organism or human body entail gradually changing pharmacokinetic concentration profiles. As concentration profile dynamics can influence drug effects on the target tissues, it is important to be able to reproduce realistic concentration profiles in in vitro systems. We present a novel design that can be integrated in tubing-free, microfluidic culture chips. These chips are actuated by tilting so that gravity-driven flow and perfusion of culture chambers can be established between reservoirs at both ends of a microfluidic channel. The design enables the realization of in vivo-like substance exposure scenarios. Compound gradients are generated through an asymmetric Y-junction of channels with different hydrodynamic resistances. Six microtissues (MTs) can be cultured and exposed in compartments along the channel. Changes of the chip design or operation parameters enable to alter the dosing profile over a large range. Modulation of, e.g., the tilting angle, changes the slope of the dosing curves, so that concentration curves can be attained that resemble the pharmacokinetic characteristics of common substances in a human body. Human colorectal cancer (HCT 116) MTs were exposed to both, gradually decreasing and constant concentrations of Staurosporine. Measurements of apoptosis induction and viability after 5 h and 24 h showed different short- and long-term responses of the MTs to dynamic and linear dosing regimes.
Keywords: drug dosing; microfluidics; microtissues; pharmacokinetics; tilting chip.
Figures


References
-
- Bruus H. (2008). Theoretical Microfluidics. Oxford: Oxford University Press.
LinkOut - more resources
Full Text Sources
Research Materials

