Telomerase Deficiency Predisposes to Heart Failure and Ischemia-Reperfusion Injury
- PMID: 31001540
- PMCID: PMC6454001
- DOI: 10.3389/fcvm.2019.00031
Telomerase Deficiency Predisposes to Heart Failure and Ischemia-Reperfusion Injury
Abstract
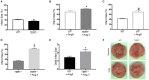
Introduction: Elevated levels of mitochondrial reactive oxygen species (ROS) contribute to the development of numerous cardiovascular diseases. TERT, the catalytic subunit of telomerase, has been shown to translocate to mitochondria to suppress ROS while promoting ATP production. Acute overexpression of TERT increases survival and decreases infarct size in a mouse model of myocardial infarct, while decreased telomerase activity predisposes to mitochondrial defects and heart failure. In the present study, we examined the role of TERT on cardiac structure and function under basal conditions and conditions of acute or prolonged stress in a novel rat model of TERT deficiency. Methods: Cardiac structure and function were evaluated via transthoracic echocardiogram. Langendorff preparations were used to test the effects of acute global ischemia reperfusion injury on cardiac function and infarction. Coronary flow and left ventricular pressure were measured during and after ischemia/reperfusion (I/R). Mitochondrial DNA integrity was measured by PCR and mitochondrial respiration was assessed in isolated mitochondria using an Oxygraph. Angiotensin II infusion was used as an established model of systemic stress. Results: No structural changes (echocardiogram) or coronary flow/left ventricle pressure (isolated hearts) were observed in TERT-/- rats at baseline; however, after I/R, coronary flow was significantly reduced in TERT-/- compared to wild type (WT) rats, while diastolic Left Ventricle Pressure was significantly elevated (n = 6 in each group; p < 0.05) in the TERT-/-. Interestingly, infarct size was less in TERT-/- rats compared to WT rats, while mitochondrial respiratory control index decreased and mitochondrial DNA lesions increased in TERT-/- compared to WT. Angiotensin II treatment did not alter cardiac structure or function; however, it augmented the infarct size significantly more in TERT-/- compared to the WT. Conclusion: Absence of TERT activity increases susceptibility to stress like cardiac injury. These results suggest a critical role of telomerase in chronic heart disease.
Keywords: heart disease; ischema-reperfusion injury; mitochondia; reactive oxygen species; telomerase (TERT).
Figures




References
-
- Gorina Y, Goulding MR, Hoyert DL, Lentzner HR. Trends in causes of death among older persons in the United States. Aging Trends. (2005) 6:1–12. - PubMed
-
- Association AH. Coronary artery disease-coronary heart disease. Dallas TX: American Heart Association; (2014).
Grants and funding
LinkOut - more resources
Full Text Sources

