Immunobiotics Beneficially Modulate TLR4 Signaling Triggered by Lipopolysaccharide and Reduce Hepatic Steatosis In Vitro
- PMID: 31001563
- PMCID: PMC6437725
- DOI: 10.1155/2019/3876896
Immunobiotics Beneficially Modulate TLR4 Signaling Triggered by Lipopolysaccharide and Reduce Hepatic Steatosis In Vitro
Abstract
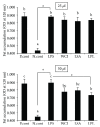
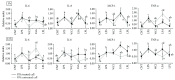
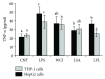
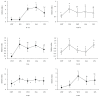
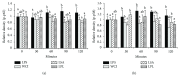
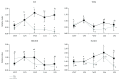
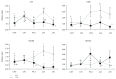
Hepatic inflammation and injury may result from the translocation of pathological bacteria and their proinflammatory mediators. Probiotics attenuate hepatic diseases related to inflammation by exhibiting immunoregulatory effects. Therefore, this study was conducted to evaluate lipid reduction and immunoregulatory potentials of probiotic bacteria in vitro. HepG2 cells treated with total cellular fluid (TCF) of LABs reduced lipid accumulation. Moreover, cells responded to lipopolysaccharide (LPS) by producing higher levels of IL-6, IL-8, MCP-1, and TNF-α. TCF of LABs treatment showed remarkably diminished levels of the expression of these cytokines via modulation of the expression of TLR-negative regulators, as well as MAPK and NF-κB pathways. Moreover, heat-killed LABs were able to diminish TGF-β, IL-1β, and IL-6 and to increase IL-10 and TLR4 levels in THP-1 cells. LABs also decreased the protein level of TNF-α. These results demonstrated that immunobiotics exhibit potent immunoregulatory activity and may be used as effective therapeutic agents to alleviate inflammatory response.
Figures
References
-
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food. Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; 2002. https://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

