Theoretical optimization of high-frequency optogenetic spiking of red-shifted very fast-Chrimson-expressing neurons
- PMID: 31001567
- PMCID: PMC6458485
- DOI: 10.1117/1.NPh.6.2.025002
Theoretical optimization of high-frequency optogenetic spiking of red-shifted very fast-Chrimson-expressing neurons
Abstract
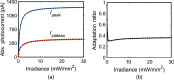
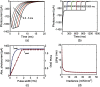
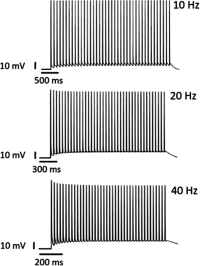
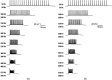
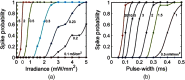
A detailed theoretical analysis and optimization of high-fidelity, high-frequency firing of the red-shifted very-fast-Chrimson (vf-Chrimson) expressing neurons is presented. A four-state model for vf-Chrimson photocycle has been formulated and incorporated in Hodgkin-Huxley and Wang-Buzsaki spiking neuron circuit models. The effect of various parameters that include irradiance, pulse width, frequency, expression level, and membrane capacitance has been studied in detail. Theoretical simulations are in excellent agreement with recently reported experimental results. The analysis and optimization bring out additional interesting features. A minimal pulse width of 1.7 ms at induces a peak photocurrent of 1250 pA. Optimal irradiance ( ) and pulse width ( ) to trigger action potential have been determined. At frequencies beyond 200 Hz, higher values of expression level and irradiance result in spike failure. Singlet and doublet spiking fidelity can be maintained up to 400 and 150 Hz, respectively. The combination of expression level and membrane capacitance is a crucial factor to achieve high-frequency firing above 500 Hz. Its optimization enables 100% spike probability of up to 1 kHz. The study is useful in designing new high-frequency optogenetic neural spiking experiments with desired spatiotemporal resolution, by providing insights into the temporal spike coding, plasticity, and curing neurodegenerative diseases.
Keywords: channelrhodopsins; computational optogenetics; neural spiking; red-shifted opsins; very-fast-Chrimson.
Figures










Similar articles
-
Theoretical Analysis of Low-power Bidirectional Optogenetic Control of High-frequency Neural Codes with Single Spike Resolution.Neuroscience. 2020 Nov 21;449:165-188. doi: 10.1016/j.neuroscience.2020.09.022. Epub 2020 Sep 15. Neuroscience. 2020. PMID: 32941934
-
Comparison of low-power, high-frequency and temporally precise optogenetic inhibition of spiking in NpHR, eNpHR3.0 and Jaws-expressing neurons.Biomed Phys Eng Express. 2020 May 20;6(4):045011. doi: 10.1088/2057-1976/ab90a1. Biomed Phys Eng Express. 2020. PMID: 33444272
-
Theoretical analysis of low-power fast optogenetic control of firing of Chronos-expressing neurons.Neurophotonics. 2018 Apr;5(2):025009. doi: 10.1117/1.NPh.5.2.025009. Epub 2018 May 24. Neurophotonics. 2018. PMID: 29845088 Free PMC article.
-
Co-expressing fast channelrhodopsin with step-function opsin overcomes spike failure due to photocurrent desensitization in optogenetics: a theoretical study.J Neural Eng. 2022 Apr 6;19(2). doi: 10.1088/1741-2552/ac6061. J Neural Eng. 2022. PMID: 35320791
-
Electric field-induced effects on neuronal cell biology accompanying dielectrophoretic trapping.Adv Anat Embryol Cell Biol. 2003;173:III-IX, 1-77. doi: 10.1007/978-3-642-55469-8. Adv Anat Embryol Cell Biol. 2003. PMID: 12901336 Review.
Cited by
-
Red Light Optogenetics in Neuroscience.Front Cell Neurosci. 2022 Jan 3;15:778900. doi: 10.3389/fncel.2021.778900. eCollection 2021. Front Cell Neurosci. 2022. PMID: 35046775 Free PMC article. Review.
-
Flexible modeling of large-scale neural network stimulation: electrical and optical extensions to The Virtual Electrode Recording Tool for EXtracellular Potentials (VERTEX).bioRxiv [Preprint]. 2025 Jan 15:2024.08.20.608687. doi: 10.1101/2024.08.20.608687. bioRxiv. 2025. Update in: J Neurosci Methods. 2025 Oct;422:110514. doi: 10.1016/j.jneumeth.2025.110514. PMID: 39229104 Free PMC article. Updated. Preprint.
-
Flexible modeling of large-scale neural network stimulation: Electrical and optical extensions to The Virtual Electrode Recording Tool for EXtracellular Potentials (VERTEX).J Neurosci Methods. 2025 Oct;422:110514. doi: 10.1016/j.jneumeth.2025.110514. Epub 2025 Jun 13. J Neurosci Methods. 2025. PMID: 40517845
-
Bridging model and experiment in systems neuroscience with Cleo: the Closed-Loop, Electrophysiology, and Optophysiology simulation testbed.bioRxiv [Preprint]. 2024 Jul 9:2023.01.27.525963. doi: 10.1101/2023.01.27.525963. bioRxiv. 2024. PMID: 39026717 Free PMC article. Preprint.
-
Theoretical analysis of low-power deep synergistic sono-optogenetic excitation of neurons by co-expressing light-sensitive and mechano-sensitive ion-channels.Commun Biol. 2025 Mar 6;8(1):379. doi: 10.1038/s42003-025-07792-8. Commun Biol. 2025. PMID: 40050670 Free PMC article.

