Preparation and Evaluation of Liposomes Co-Loaded with Doxorubicin, Phospholipase D Inhibitor 5-Fluoro-2-Indolyl Deschlorohalopemide (FIPI) and D-Alpha Tocopheryl Acid Succinate (α-TOS) for Anti-Metastasis
- PMID: 31001703
- PMCID: PMC6473021
- DOI: 10.1186/s11671-019-2964-4
Preparation and Evaluation of Liposomes Co-Loaded with Doxorubicin, Phospholipase D Inhibitor 5-Fluoro-2-Indolyl Deschlorohalopemide (FIPI) and D-Alpha Tocopheryl Acid Succinate (α-TOS) for Anti-Metastasis
Abstract
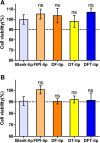
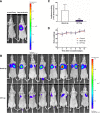
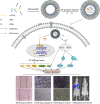
Tumor metastasis has become a key obstacle to cancer treatment, which causes high mortality. Nowadays, it involves multiple complex pathways, and conventional treatments are not effective due to fewer targets. The aims of the present study were to construct a novel liposome delivery system co-loading a specific PLD inhibitor 5-fluoro-2-indolyldes-chlorohalopemide (FIPI) in combination with antitumor drug doxorubicin (DOX) and functional excipient D-alpha tocopheryl acid succinate (α-TOS) for anti-metastasis. In this study, the liposomes containing three components (DFT-Lip) with different physicochemical properties were successfully prepared by film dispersion method combined with pH-gradient method. Physicochemical parameters such as particles size, potential, encapsulation efficiency, stability, and release profiles were investigated. In vitro and in vivo anti-metastasis effectiveness against highly metastatic breast cancer MDA-MB-231 cell line was evaluated. The liposomes showed uniform particle size (approximately 119 nm), high drug encapsulation efficiency (> 90%), slow release characteristics and stability. In vitro anti-tumor cell metastasis study demonstrated DFT-Lip could greatly inhibit motility, migration and invasion of MDA-MB-231 cells compared to other liposomes, predicting a synergistic anti-tumor metastasis effect between FIPI with α-TOS in liposomes. In vivo anti-metastasis study showed that DFT-Lip prevented the initiation and the progression of metastasis of high metastatic breast cancer. These results suggested that the liposomes containing DOX, FIPI, and α-TOS might be a promising strategy for metastatic tumor therapy in clinics.
Keywords: Anti-metastasis; DOX; FIPI; Liposomes; α-TOS.
Conflict of interest statement
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
Co-delivery nanocarriers targeting folate receptor and encapsulating 2-deoxyglucose and α-tocopheryl succinate enhance anti-tumor effect in vivo.Int J Nanomedicine. 2017 Aug 8;12:5701-5715. doi: 10.2147/IJN.S135849. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28848348 Free PMC article.
-
Co-delivery of doxorubicin and PSC 833 (Valspodar) by stealth nanoliposomes for efficient overcoming of multidrug resistance.J Pharm Pharm Sci. 2012;15(4):568-82. doi: 10.18433/j3sc7j. J Pharm Pharm Sci. 2012. PMID: 23106959
-
A liposome-based combination strategy using doxorubicin and a PI3K inhibitor efficiently inhibits pre-metastatic initiation by acting on both tumor cells and tumor-associated macrophages.Nanoscale. 2022 Mar 24;14(12):4573-4587. doi: 10.1039/d1nr08215a. Nanoscale. 2022. PMID: 35253829
-
pH-sensitive polymeric nanoparticles for co-delivery of doxorubicin and curcumin to treat cancer via enhanced pro-apoptotic and anti-angiogenic activities.Acta Biomater. 2017 Aug;58:349-364. doi: 10.1016/j.actbio.2017.04.029. Epub 2017 Apr 26. Acta Biomater. 2017. PMID: 28455219
-
Vitamin E D-alpha-tocopheryl polyethylene glycol 1000 succinate-conjugated liposomal docetaxel reverses multidrug resistance in breast cancer cells.J Pharm Pharmacol. 2019 Aug;71(8):1243-1254. doi: 10.1111/jphp.13126. Epub 2019 Jun 18. J Pharm Pharmacol. 2019. PMID: 31215039
Cited by
-
Fabrication of 3D-Printed Fish-Gelatin-Based Polymer Hydrogel Patches for Local Delivery of PEGylated Liposomal Doxorubicin.Mar Drugs. 2020 Jun 20;18(6):325. doi: 10.3390/md18060325. Mar Drugs. 2020. PMID: 32575787 Free PMC article.
-
Doxorubicin-Loaded Gold Nanoarchitectures as a Therapeutic Strategy against Diffuse Intrinsic Pontine Glioma.Cancers (Basel). 2021 Mar 13;13(6):1278. doi: 10.3390/cancers13061278. Cancers (Basel). 2021. PMID: 33805713 Free PMC article.
-
Application of Density Functional Theory to Molecular Engineering of Pharmaceutical Formulations.Int J Mol Sci. 2025 Apr 1;26(7):3262. doi: 10.3390/ijms26073262. Int J Mol Sci. 2025. PMID: 40244098 Free PMC article. Review.
-
Preparation, Pharmacokinetics, and Antitumor Potential of Miltefosine-Loaded Nanostructured Lipid Carriers.Int J Nanomedicine. 2021 May 11;16:3255-3273. doi: 10.2147/IJN.S299443. eCollection 2021. Int J Nanomedicine. 2021. Retraction in: Int J Nanomedicine. 2023 Mar 01;18:1107-1108. doi: 10.2147/IJN.S409956. PMID: 34012260 Free PMC article. Retracted.
References
-
- Ahmad O, Chan M, Savage P, Watabe K, Lo HW, Qasem S. Biology and treatment of metastasis of sarcoma to the brain. Front Biosci (Elite Ed) 2016;8:233–244. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

