Altered donor P2X7 activity in human leukocytes correlates with P2RX7 genotype but does not affect the development of graft-versus-host disease in humanised mice
- PMID: 31001750
- PMCID: PMC6635536
- DOI: 10.1007/s11302-019-09651-8
Altered donor P2X7 activity in human leukocytes correlates with P2RX7 genotype but does not affect the development of graft-versus-host disease in humanised mice
Abstract
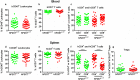
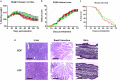
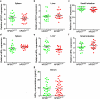
Graft-versus-host disease (GVHD) is a life-threatening consequence of allogeneic haematopoietic stem cell transplantation, a curative therapy for haematological malignancies. The ATP-gated P2X7 receptor channel is implicated in the development of GVHD. P2X7 activity on human leukocytes can be influenced by gain-of-function (GOF) and loss-of-function (LOF) single nucleotide polymorphisms (SNPs) in the P2RX7 gene. In this study, the P2RX7 gene was sequenced in 25 human donors and the P2X7 activity on subsets of peripheral blood T cells, natural killer (NK) cells and monocytes was measured using an ATP-induced dye uptake assay. GOF and LOF SNPs representing 10 of the 17 known P2RX7 haplotypes were identified, and correlated with P2X7 activity on all leukocyte subsets investigated. Notably, invariant (i) NK T cells displayed the highest P2X7 activity amongst all cell types studied. To determine if donor P2X7 activity influenced the development of GVHD, immunodeficient NOD-SCID-IL2Rγnull (NSG) mice were injected with human peripheral blood mononuclear cells isolated from donors of either GOF (hP2X7GOF mice) or LOF (hP2X7LOF mice) P2RX7 genotype. Both hP2X7GOF and hP2X7LOF mice demonstrated similar human leukocyte engraftment, and showed comparable weight loss, GVHD clinical score and overall survival. Donor P2X7 activity did not affect human leukocyte infiltration or GVHD-mediated tissue damage, or the relative expression of human P2X7 or human interferon-γ (hIFNγ) in tissues. Finally, hP2X7GOF and hP2X7LOF mice demonstrated similar concentrations of serum hIFNγ. This study demonstrates that P2X7 activity correlates with donor P2RX7 genotype on human leukocyte subsets important in GVHD development, but does not affect GVHD development in a humanised mouse model of this disease.
Keywords: Bone marrow transplantation; Humanised mice; Leukocyte; P2RX7 gene single nucleotide polymorphism; P2X7 receptor; Purinergic receptor; Xenogeneic graft-versus-host disease.
Conflict of interest statement
Sam R Adhikary declares that he has no conflict of interest.
Nicholas J Geraghty declares that he has no conflict of interest.
Peter Cuthbertson declares that he has no conflict of interest.
Ronald Sluyter declares that he has no conflict of interest.
Debbie Watson declares that she has no conflict of interest.
Figures


Similar articles
-
Increased P2X7 expression in the gastrointestinal tract and skin in a humanised mouse model of graft-versus-host disease.Clin Sci (Lond). 2020 Jan 31;134(2):207-223. doi: 10.1042/CS20191086. Clin Sci (Lond). 2020. PMID: 31934722
-
The P2X7 receptor antagonist Brilliant Blue G reduces serum human interferon-γ in a humanized mouse model of graft-versus-host disease.Clin Exp Immunol. 2017 Oct;190(1):79-95. doi: 10.1111/cei.13005. Epub 2017 Jul 24. Clin Exp Immunol. 2017. PMID: 28665482 Free PMC article.
-
A Species-Specific Anti-Human P2X7 Monoclonal Antibody Reduces Graft-versus-Host Disease in Humanised Mice.Pharmaceutics. 2023 Aug 31;15(9):2263. doi: 10.3390/pharmaceutics15092263. Pharmaceutics. 2023. PMID: 37765233 Free PMC article.
-
Insights into mechanisms of graft-versus-host disease through humanised mouse models.Biosci Rep. 2022 Sep 30;42(9):BSR20211986. doi: 10.1042/BSR20211986. Biosci Rep. 2022. PMID: 35993192 Free PMC article. Review.
-
Purinergic signalling in graft-versus-host disease.Curr Opin Pharmacol. 2023 Feb;68:102346. doi: 10.1016/j.coph.2022.102346. Epub 2023 Jan 10. Curr Opin Pharmacol. 2023. PMID: 36634595 Review.
Cited by
-
Purinergic Signalling in Allogeneic Haematopoietic Stem Cell Transplantation and Graft-versus-Host Disease.Int J Mol Sci. 2021 Aug 3;22(15):8343. doi: 10.3390/ijms22158343. Int J Mol Sci. 2021. PMID: 34361109 Free PMC article. Review.
-
P2X7 Receptor in Dendritic Cells and Macrophages: Implications in Antigen Presentation and T Lymphocyte Activation.Int J Mol Sci. 2024 Feb 21;25(5):2495. doi: 10.3390/ijms25052495. Int J Mol Sci. 2024. PMID: 38473744 Free PMC article. Review.
-
Animal Models for the Investigation of P2X7 Receptors.Int J Mol Sci. 2023 May 4;24(9):8225. doi: 10.3390/ijms24098225. Int J Mol Sci. 2023. PMID: 37175933 Free PMC article. Review.
-
Graft-versus-leukaemia immunity is retained following treatment with post-transplant cyclophosphamide alone or combined with tocilizumab in humanised mice.Clin Transl Immunology. 2024 Mar 15;13(3):e1497. doi: 10.1002/cti2.1497. eCollection 2024. Clin Transl Immunology. 2024. PMID: 38495918 Free PMC article.
-
Humanized Mouse Model of Graft-Versus-Host Disease Utilizing Nonirradiated NOD-scid-IL-2Rγnull Mice.Methods Mol Biol. 2025;2907:183-206. doi: 10.1007/978-1-0716-4430-0_9. Methods Mol Biol. 2025. PMID: 40100599
References
-
- Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90:3204–3213. - PubMed
-
- Storb R, Gyurkocza B, Storer BE, Sorror ML, Blume K, Niederwieser D, Chauncey TR, Pulsipher MA, Petersen FB, Sahebi F, Agura ED, Hari P, Bruno B, McSweeney PA, Maris MB, Maziarz RT, Langston AA, Bethge W, Vindelov L, Franke GN, Laport GG, Yeager AM, Hubel K, Deeg HJ, Georges GE, Flowers ME, Martin PJ, Mielcarek M, Woolfrey AE, Maloney DG, Sandmaier BM. Graft-versus-host disease and graft-versus-tumor effects after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2013;31:1530–1538. doi: 10.1200/JCO.2012.45.0247. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

