Evaluation of in vitro hemolysis and platelet activation of a newly developed maglev LVAD and two clinically used LVADs with human blood
- PMID: 31001834
- PMCID: PMC6733624
- DOI: 10.1111/aor.13471
Evaluation of in vitro hemolysis and platelet activation of a newly developed maglev LVAD and two clinically used LVADs with human blood
Abstract
In vitro hemolysis testing remains one of the most important performance measures to judge the hemocompatibility of a left ventricular assist device (LVAD). Clinically relevant operating conditions and appropriate testing blood are essential to infer in vitro data for potential clinical use. This in vitro study was carried out to evaluate and compare the hemolytic performance of a newly developed magnetically levitated (maglev) LVAD (CH-VAD) with two clinically used LVADs (HVAD and HeartMate II (HMII)) using fresh human blood. A small volume (~300 mL) in vitro circulating flow loop was constructed with a LVAD generated flow of 4.5 L/min at the nominal or reported clinical operating speed for each LVAD. The blood was circulated in the loop for 4 hours with samples drawn at baseline and hourly. Plasma-free hemoglobin (PFH) concentrations in the hourly blood samples were determined with spectrophotometry. Normalized index of hemolysis (NIH) was calculated to compare the hemolytic performance of the CH-VAD and the two reference LVADs. Platelet activation was measured with flow cytometry. The experimental test for each device was repeated at least 7 times. The data from this study showed that all the three LVADs generated very low hemolysis (NIH <0.01 g/100 L). The CH-VAD was found to have a significantly lower NIH value (0.00135 ± 0.00032 g/100 L) compared to the HVAD (0.00525 ± 0.00183 g/100 L) and the HMII (0.00583 ± 0.00182 g/100 L). No statistically significant difference in device-generated hemolysis was found between the HVAD and the HMII. The level of platelet activation induced by the CH-VAD is significantly lower than those by the HVAD and the HMII. The data suggest that the shear-induced hemolysis and platelet activation of the CH-VAD are acceptable relative to the two LVADs currently in clinical use.
Keywords: hemocompatibility; hemolysis; left ventricular assist device; magnetically levitated blood pump.
© 2019 International Center for Artificial Organs and Transplantation and Wiley Periodicals, Inc.
Conflict of interest statement
Declaration of Interest statement
All authors declared no conflicts of interests.
Figures





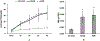
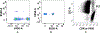
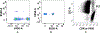
References
-
- Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart Disease and Stroke Statistics - 2018 Update. A Report from the American Heart Association. Circulation 2018;137:e67–e492. - PubMed
-
- Organ Procurement and Transplantation Network (OPTN) by the U.S. Department of Health & Human Services (https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/# accessed on Dec 04, 2018).
-
- Colvin M, Smith JM, Hadley N, Skeans MA, Carrico R, Uccellini K, et al. OPTN/SRTR 2016 Annual Data Report: Heart. Am J Transplant 2018;18 Suppl 1:291–362. - PubMed
-
- Starling RC, Naka Y, Boyle AJ, Gonzalez-Stawinski G, John R, Jorde U, et al. Results of the post-U.S. Food and Drug Administration-approval study with a continuous flow left ventricular assist device as a bridge to heart transplantation: a prospective study using the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support). J Am Coll Cardiol 2011;57(19):1890–8. - PubMed
-
- Strueber M, O’Driscoll G, Jansz P, Khaghani A, Levy WC, Wieselthaler GM, et al. Multicenter evaluation of an intrapericardial left ventricular assist system. J Am Coll Cardiol 2011;57(12):1375–82. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

