The transcription factors ActR and SoxR differentially affect the phenazine tolerance of Agrobacterium tumefaciens
- PMID: 31001852
- PMCID: PMC6615960
- DOI: 10.1111/mmi.14263
The transcription factors ActR and SoxR differentially affect the phenazine tolerance of Agrobacterium tumefaciens
Abstract
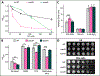
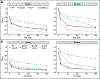
Bacteria in soils encounter redox-active compounds, such as phenazines, that can generate oxidative stress, but the mechanisms by which different species tolerate these compounds are not fully understood. Here, we identify two transcription factors, ActR and SoxR, that play contrasting yet complementary roles in the tolerance of the soil bacterium Agrobacterium tumefaciens to phenazines. We show that ActR promotes phenazine tolerance by proactively driving expression of a more energy-efficient terminal oxidase at the expense of a less efficient alternative, which may affect the rate at which phenazines abstract electrons from the electron transport chain (ETC) and thereby generate reactive oxygen species. SoxR, on the other hand, responds to phenazines by inducing expression of several efflux pumps and redox-related genes, including one of three copies of superoxide dismutase and five novel members of its regulon that could not be computationally predicted. Notably, loss of ActR is far more detrimental than loss of SoxR at low concentrations of phenazines, and also increases dependence on the otherwise functionally redundant SoxR-regulated superoxide dismutase. Our results thus raise the intriguing possibility that the composition of an organism's ETC may be the driving factor in determining sensitivity or tolerance to redox-active compounds.
© 2019 John Wiley & Sons Ltd.
Figures





Similar articles
-
Pseudomonas aeruginosa PumA acts on an endogenous phenazine to promote self-resistance.Microbiology (Reading). 2018 May;164(5):790-800. doi: 10.1099/mic.0.000657. Epub 2018 Apr 9. Microbiology (Reading). 2018. PMID: 29629858 Free PMC article.
-
Agrobacterium tumefaciens soxR is involved in superoxide stress protection and also directly regulates superoxide-inducible expression of itself and a target gene.J Bacteriol. 2006 Dec;188(24):8669-73. doi: 10.1128/JB.00856-06. Epub 2006 Oct 13. J Bacteriol. 2006. PMID: 17041041 Free PMC article.
-
Two-stage control of an oxidative stress regulon: the Escherichia coli SoxR protein triggers redox-inducible expression of the soxS regulatory gene.J Bacteriol. 1992 Oct;174(19):6054-60. doi: 10.1128/jb.174.19.6054-6060.1992. J Bacteriol. 1992. PMID: 1400156 Free PMC article.
-
Sensing and protecting against superoxide stress in Escherichia coli--how many ways are there to trigger soxRS response?Redox Rep. 2000;5(5):287-93. doi: 10.1179/135100000101535825. Redox Rep. 2000. PMID: 11145103 Review.
-
Redox signal transduction via iron-sulfur clusters in the SoxR transcription activator.Trends Biochem Sci. 1997 Jun;22(6):207-10. doi: 10.1016/s0968-0004(97)01068-2. Trends Biochem Sci. 1997. PMID: 9204707 Review.
Cited by
-
Prevalence and Correlates of Phenazine Resistance in Culturable Bacteria from a Dryland Wheat Field.Appl Environ Microbiol. 2022 Mar 22;88(6):e0232021. doi: 10.1128/aem.02320-21. Epub 2022 Feb 9. Appl Environ Microbiol. 2022. PMID: 35138927 Free PMC article.
-
From the soil to the clinic: the impact of microbial secondary metabolites on antibiotic tolerance and resistance.Nat Rev Microbiol. 2022 Mar;20(3):129-142. doi: 10.1038/s41579-021-00620-w. Epub 2021 Sep 16. Nat Rev Microbiol. 2022. PMID: 34531577 Free PMC article. Review.
-
Global landscape of phenazine biosynthesis and biodegradation reveals species-specific colonization patterns in agricultural soils and crop microbiomes.Elife. 2020 Sep 15;9:e59726. doi: 10.7554/eLife.59726. Elife. 2020. PMID: 32930660 Free PMC article.
-
Regulation of the H1 Type VI Secretion System by the Transcriptional Regulator NfxB in Pseudomonas aeruginosa.Int J Mol Sci. 2025 Feb 10;26(4):1472. doi: 10.3390/ijms26041472. Int J Mol Sci. 2025. PMID: 40003937 Free PMC article.
-
Heat-shock proteases promote survival of Pseudomonas aeruginosa during growth arrest.Proc Natl Acad Sci U S A. 2020 Feb 25;117(8):4358-4367. doi: 10.1073/pnas.1912082117. Epub 2020 Feb 6. Proc Natl Acad Sci U S A. 2020. PMID: 32029587 Free PMC article.
References
-
- Alonso A, Rojo F, and Martínez JL (1999) Environmental and clinical isolates of Pseudomonas aeruginosa show pathogenic and biodegradative properties irrespective of their origin. Environ Microbiol 1: 421–430. - PubMed
-
- Audenaert K, Pattery T, Cornelis P, and Höfte M (2002) Induction of systemic resistance to Botrytis cinerea in tomato by Pseudomonas aeruginosa 7NSK2: role of salicylic acid, pyochelin, and pyocyanin. Mol Plant-Microbe Interact 15: 1147–1156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

