A gamma-thionin protein from apple, MdD1, is required for defence against S-RNase-induced inhibition of pollen tube prior to self/non-self recognition
- PMID: 31001872
- PMCID: PMC6790362
- DOI: 10.1111/pbi.13131
A gamma-thionin protein from apple, MdD1, is required for defence against S-RNase-induced inhibition of pollen tube prior to self/non-self recognition
Abstract
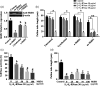
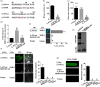
Apple exhibits S-RNase-mediated self-incompatibility. Although the cytotoxic effect of S-RNase inside the self-pollen tube has been studied extensively, the underlying defence mechanism in pollen tube in Rosaceae remains unclear. On exposure to stylar S-RNase, plant defence responses are activated in the pollen tube; however, how these are regulated is currently poorly understood. Here, we show that entry of both self and non-self S-RNase into pollen tubes of apple (Malus domestica) stimulates jasmonic acid (JA) production, in turn inducing the accumulation of MdMYC2 transcripts, a transcription factor in the JA signalling pathway widely considered to be involved in plant defence processes. MdMYC2 acts as a positive regulator in the pollen tube activating expression of MdD1, a gene encoding a defence protein. Importantly, MdD1 was shown to bind to the RNase activity sites of S-RNase leading to inhibition of enzymatic activity. This work provides intriguing insights into an ancient defence mechanism present in apple pollen tubes where MdD1 likely acts as a primary line of defence to inhibit S-RNase cytotoxicity prior to self/non-self recognition.
Keywords: Malus domestica; MdD1; S-RNase; plant defence; pollen tube growth.
© 2019 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Apple MdABCF assists in the transportation of S-RNase into pollen tubes.Plant J. 2014 Jun;78(6):990-1002. doi: 10.1111/tpj.12524. Epub 2014 May 12. Plant J. 2014. PMID: 24684704
-
Apple S-RNase interacts with an actin-binding protein, MdMVG, to reduce pollen tube growth by inhibiting its actin-severing activity at the early stage of self-pollination induction.Plant J. 2018 Jul;95(1):41-56. doi: 10.1111/tpj.13929. Epub 2018 Jun 4. Plant J. 2018. PMID: 29667261
-
Apple S-RNase triggers inhibition of tRNA aminoacylation by interacting with a soluble inorganic pyrophosphatase in growing self-pollen tubes in vitro.New Phytol. 2018 Apr;218(2):579-593. doi: 10.1111/nph.15028. Epub 2018 Feb 9. New Phytol. 2018. PMID: 29424440
-
Cytoskeleton, Transglutaminase and Gametophytic Self-Incompatibility in the Malinae (Rosaceae).Int J Mol Sci. 2019 Jan 8;20(1):209. doi: 10.3390/ijms20010209. Int J Mol Sci. 2019. PMID: 30626063 Free PMC article. Review.
-
'A life or death decision' for pollen tubes in S-RNase-based self-incompatibility.J Exp Bot. 2010 Apr;61(7):2027-37. doi: 10.1093/jxb/erp381. Epub 2009 Dec 30. J Exp Bot. 2010. PMID: 20042540 Review.
Cited by
-
PbrBZR1 interacts with PbrARI2.3 to mediate brassinosteroid-regulated pollen tube growth during self-incompatibility signaling in pear.Plant Physiol. 2023 Jul 3;192(3):2356-2373. doi: 10.1093/plphys/kiad208. Plant Physiol. 2023. PMID: 37010117 Free PMC article.
-
Transcriptome Analysis of the Late-Acting Self-Incompatibility Associated with RNase T2 Family in Camellia oleifera.Plants (Basel). 2023 May 9;12(10):1932. doi: 10.3390/plants12101932. Plants (Basel). 2023. PMID: 37653852 Free PMC article.
-
The influence of the pollination compatibility type on the pistil S-RNase expression in European pear (Pyrus communis).Front Genet. 2024 Apr 9;15:1360332. doi: 10.3389/fgene.2024.1360332. eCollection 2024. Front Genet. 2024. PMID: 38655055 Free PMC article.
-
The Mechanism of Ovule Abortion in Self-Pollinated 'Hanfu' Apple Fruits and Related Gene Screening.Plants (Basel). 2024 Mar 30;13(7):996. doi: 10.3390/plants13070996. Plants (Basel). 2024. PMID: 38611525 Free PMC article.
-
Polyamines Involved in Regulating Self-Incompatibility in Apple.Genes (Basel). 2021 Nov 15;12(11):1797. doi: 10.3390/genes12111797. Genes (Basel). 2021. PMID: 34828403 Free PMC article.
References
-
- Athukorala, S.N.P. and Piercey‐Normore, M.D. (2015) Recognition‐ and defense‐related gene expression at 3 resynthesis stages in lichen symbionts. Can. J. Microbiol. 61, 1–12. - PubMed
-
- Baxter, A. , Mittler, R. and Suzuki, N. (2014) ROS as key players in plant stress signalling. J. Exp. Bot. 65, 1229–1240. - PubMed
-
- Bircheneder, S. and Dresselhaus, T. (2016) Why cellular communication during plant reproduction is particularly mediated by CRP signalling. J. Exp. Bot. 67, 4849–4861. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

