Microbial osteoporosis: The interplay between the gut microbiota and bones via host metabolism and immunity
- PMID: 31001921
- PMCID: PMC6692530
- DOI: 10.1002/mbo3.810
Microbial osteoporosis: The interplay between the gut microbiota and bones via host metabolism and immunity
Abstract
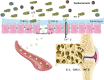
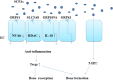
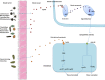
The complex relationship between intestinal microbiota and host is a novel field in recent years. A large number of studies are being conducted on the relationship between intestinal microbiota and bone metabolism. Bone metabolism consisted of bone absorption and formation exists in the whole process of human growth and development. The nutrient components, inflammatory factors, and hormone environment play important roles in bone metabolism. Recently, intestinal microbiota has been found to influence bone metabolism via influencing the host metabolism, immune function, and hormone secretion. Here, we searched relevant literature on Pubmed and reviewed the effect of intestinal microbiota on bone metabolism through the three aspects, which may provide new ideas and targets for the clinical treatment of osteoporosis.
Keywords: bone formation; bone resorption; intestinal microbiota; osteoporosis.
© 2019 The Authors. MicrobiologyOpen published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
The role of gut microbiota in bone homeostasis.Bone. 2020 Jun;135:115317. doi: 10.1016/j.bone.2020.115317. Epub 2020 Mar 10. Bone. 2020. PMID: 32169602 Free PMC article. Review.
-
The Human Gut Microbiota: A Key Mediator of Osteoporosis and Osteogenesis.Int J Mol Sci. 2021 Aug 31;22(17):9452. doi: 10.3390/ijms22179452. Int J Mol Sci. 2021. PMID: 34502371 Free PMC article. Review.
-
Association Between Gut Microbiota and Bone Health: Potential Mechanisms and Prospective.J Clin Endocrinol Metab. 2017 Oct 1;102(10):3635-3646. doi: 10.1210/jc.2017-00513. J Clin Endocrinol Metab. 2017. PMID: 28973392 Free PMC article. Review.
-
Microbiota-bone axis in ageing-related bone diseases.Front Endocrinol (Lausanne). 2024 Jul 15;15:1414350. doi: 10.3389/fendo.2024.1414350. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39076510 Free PMC article. Review.
-
Gut microbiota and bone metabolism.FASEB J. 2021 Jul;35(7):e21740. doi: 10.1096/fj.202100451R. FASEB J. 2021. PMID: 34143911 Review.
Cited by
-
Blackcurrant Anthocyanins Attenuate Estrogen -Deficiency-Induced Bone Loss through Modulating Microbial-Derived Short-Chain Carboxylic Acids and Phytoestrogen Metabolites in Peri- and Early Postmenopausal Women.Metabolites. 2024 Oct 11;14(10):541. doi: 10.3390/metabo14100541. Metabolites. 2024. PMID: 39452922 Free PMC article.
-
Extracellular vesicles derived from the periodontal pathogen Filifactor alocis induce systemic bone loss through Toll-like receptor 2.J Extracell Vesicles. 2021 Oct;10(12):e12157. doi: 10.1002/jev2.12157. J Extracell Vesicles. 2021. PMID: 34648247 Free PMC article.
-
Intermittent Parathyroid Hormone Alters Gut Microbiota in Ovariectomized Osteoporotic Rats.Orthop Surg. 2022 Sep;14(9):2330-2338. doi: 10.1111/os.13419. Epub 2022 Aug 10. Orthop Surg. 2022. PMID: 35946436 Free PMC article.
-
Pivotal Role of Intestinal Microbiota and Intraluminal Metabolites for the Maintenance of Gut-Bone Physiology.Int J Mol Sci. 2023 Mar 8;24(6):5161. doi: 10.3390/ijms24065161. Int J Mol Sci. 2023. PMID: 36982235 Free PMC article. Review.
-
Gut microbial-derived short-chain fatty acids and bone: a potential role in fracture healing.Eur Cell Mater. 2021 Apr 21;41:454-470. doi: 10.22203/eCM.v041a29. Eur Cell Mater. 2021. PMID: 33881768 Free PMC article. Review.
References
-
- Adeel, S. , Singh, K. , Vydareny, K. H. , Kumari, M. , Shah, E. , Weitzmann, M. N. , & Tangpricha, V. (2013). Bone loss in surgically ovariectomized premenopausal women is associated with T lymphocyte activation and thymic hypertrophy. Journal of Investigative Medicine, 61, 1178–1183. 10.2310/JIM.0000000000000016 - DOI - PMC - PubMed
-
- Asano, Y. , Hiramoto, T. , Nishino, R. , Aiba, Y. , Kimura, T. , Yoshihara, K. , … Sudo, N. (2012). Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. American Journal of Physiology Gastrointestinal & Liver Physiology, 303, G1288–G1295. 10.1152/ajpgi.00341.2012 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

