Predictors of reversible airway obstruction with omalizumab in severe asthma: a real-life study
- PMID: 31002021
- PMCID: PMC6475845
- DOI: 10.1177/1753466619841274
Predictors of reversible airway obstruction with omalizumab in severe asthma: a real-life study
Abstract
Background: Omalizumab may modulate airway remodeling in severe asthma. Using forced expiratory volume in 1 second (FEV1) as a surrogate of airway remodeling, we aimed to investigate if an omalizumab add-on in severe allergic asthma may lead to a persistent reversal of airway obstruction and to evaluate the potential biomarkers of airway obstruction reversibility.
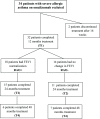
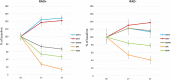
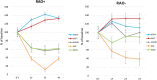
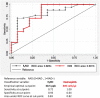
Methods: Data were collected before (T0) and after omalizumab add-on for 1 year (T1, 32 patients), 2 years (T2, 26 patients) and 4 years (T4, 13 patients). All patients had baseline FEV1 below 80 % predicted (60.5 ± 12.5 %). After omalizumab, 18 patients showed FEV1 normalization (reversible airway obstruction; RAO+) already at T1 (88.7 ± 14.9 %, p < 0.0001) that persisted up to T4 (83.2 ± 7.9, p < 0.01), while 14 patients (RAO-) had FEV1 persistently decreased, from T1 (65.2 ± 8.4%, p < 0.05) up to T4 (61.4 ± 6.2%, not significant). Both groups had significant improvement of symptoms and exacerbations after omalizumab at T1, which persisted up to T4. The comparison between pretreatment characteristics of the two groups showed that RAO+ patients, had higher values of circulating eosinophils, exhaled nitric oxide (FENO), prevalence of rhinitis and nasal polyps, need of oral corticosteroids, shorter asthma duration, higher FEV1 and response to albuterol test. The optimal cut-off points predicting FEV1 normalization after omalizumab add-on were 30.5 ppb for FENO and 305 cells/µl for eosinophils.
Conclusions: This study suggests that omalizumab add-on contributes to the persistent reversal of airway obstruction in a consistent number of patients with severe allergic asthma, and this beneficial effect is predicted by elevated pretreatment FENO and circulating eosinophils.
Keywords: FNO; airway obstruction reversibility; circulating eosinophils; omalizumab; severe allergic asthma.
Conflict of interest statement
Figures
Similar articles
-
Retrospective Study on the Association of Biomarkers With Real-world Outcomes of Omalizumab-treated Patients With Allergic Asthma.Clin Ther. 2019 Oct;41(10):1956-1971. doi: 10.1016/j.clinthera.2019.07.021. Epub 2019 Sep 25. Clin Ther. 2019. PMID: 31563391
-
Longterm clinical outcomes of omalizumab therapy in severe allergic asthma: Study of efficacy and safety.Respir Med. 2017 Mar;124:36-43. doi: 10.1016/j.rmed.2017.01.008. Epub 2017 Jan 25. Respir Med. 2017. PMID: 28284319
-
Omalizumab lowers asthma exacerbations, oral corticosteroid intake and blood eosinophils: Results of a 5-YEAR single-centre observational study.Pulm Pharmacol Ther. 2019 Feb;54:25-30. doi: 10.1016/j.pupt.2018.11.002. Epub 2018 Nov 7. Pulm Pharmacol Ther. 2019. PMID: 30414440
-
Factors reducing omalizumab response in severe asthma.Eur J Intern Med. 2018 Jun;52:78-85. doi: 10.1016/j.ejim.2018.01.026. Epub 2018 Feb 1. Eur J Intern Med. 2018. PMID: 29395935
-
Mechanisms Mediating Pediatric Severe Asthma and Potential Novel Therapies.Front Pediatr. 2017 Jul 5;5:154. doi: 10.3389/fped.2017.00154. eCollection 2017. Front Pediatr. 2017. PMID: 28725641 Free PMC article. Review.
Cited by
-
Upper airway disease diagnosis as a predictive biomarker of therapeutic response to biologics in severe asthma.Front Med (Lausanne). 2023 Mar 22;10:1129300. doi: 10.3389/fmed.2023.1129300. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37035303 Free PMC article. Review.
-
Effects of omalizumab on lung function in patients with moderate-to-severe allergic asthma: a systematic review and meta-analysis.Ther Adv Respir Dis. 2024 Jan-Dec;18:17534666231221771. doi: 10.1177/17534666231221771. Ther Adv Respir Dis. 2024. PMID: 38235607 Free PMC article.
-
Effectiveness of omalizumab in patients with severe allergic asthma with and without chronic rhinosinusitis with nasal polyps: a PROXIMA study post hoc analysis.Clin Transl Allergy. 2020 Jun 26;10:25. doi: 10.1186/s13601-020-00330-1. eCollection 2020. Clin Transl Allergy. 2020. PMID: 32607141 Free PMC article.
-
Comparison between clinical trials and real-world evidence studies on biologics for severe asthma.J Int Med Res. 2022 Nov;50(11):3000605221133689. doi: 10.1177/03000605221133689. J Int Med Res. 2022. PMID: 36420737 Free PMC article. Review.
-
Research advances in airway remodeling in asthma: a narrative review.Ann Transl Med. 2022 Sep;10(18):1023. doi: 10.21037/atm-22-2835. Ann Transl Med. 2022. PMID: 36267708 Free PMC article. Review.
References
-
- Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343–373. - PubMed
-
- Global Initiative for Asthma. GINA, http://ginasthma.org/ Accessed 22 January 2018.
-
- Presta L, Shields R, O’Connell L, et al. The binding site on human immunoglobulin E for its high affinity receptor. J Biol Chem 1994; 269: 26368–26373. - PubMed
-
- Milgrom H, Fick RB, Jr, Su JQ, et al. Treatment of allergic asthma with monoclonal anti-IgE antibody. rhuMAb-E25 Study Group. N Engl J Med 1999; 341: 1966–1973. - PubMed
-
- Bousquet J, Wenzel S, Holgate S, et al. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest 2004; 125: 1378–1386. - PubMed

